Single-cell force spectroscopy of the medically important Staphylococcus epidermidis-Candida albicans interaction
- PMID: 24057018
- PMCID: PMC3825105
- DOI: 10.1039/c3nr03272h
Single-cell force spectroscopy of the medically important Staphylococcus epidermidis-Candida albicans interaction
Abstract
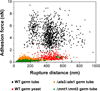
Despite the clinical importance of bacterial-fungal interactions, their molecular details are poorly understood. A hallmark of such medically important interspecies associations is the interaction between the two nosocomial pathogens Staphylococcus aureus and Candida albicans, which can lead to mixed biofilm-associated infections with enhanced antibiotic resistance. Here, we use single-cell force spectroscopy (SCFS) to quantify the forces engaged in bacterial-fungal co-adhesion, focusing on the poorly investigated S. epidermidis-C. albicans interaction. Force curves recorded between single bacterial and fungal germ tubes showed large adhesion forces (~5 nN) with extended rupture lengths (up to 500 nm). By contrast, bacteria poorly adhered to yeast cells, emphasizing the important role of the yeast-to-hyphae transition in mediating adhesion to bacterial cells. Analysis of mutant strains altered in cell wall composition allowed us to distinguish the main fungal components involved in adhesion, i.e. Als proteins and O-mannosylations. We suggest that the measured co-adhesion forces are involved in the formation of mixed biofilms, thus possibly as well in promoting polymicrobial infections. In the future, we anticipate that this SCFS platform will be used in nanomedicine to decipher the molecular mechanisms of a wide variety of pathogen-pathogen interactions and may help in designing novel anti-adhesion agents.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

