Prednisolone improves walking in Japanese Duchenne muscular dystrophy patients
- PMID: 24057148
- PMCID: PMC3843366
- DOI: 10.1007/s00415-013-7104-y
Prednisolone improves walking in Japanese Duchenne muscular dystrophy patients
Abstract
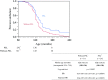
We evaluated the long-term efficacy of prednisolone (PSL) therapy for prolonging ambulation in Japanese patients with genetically confirmed Duchenne muscular dystrophy (DMD). There were clinical trials have shown a short-term positive effect of high-dose and daily PSL on ambulation, whereas a few study showed a long-term effect. Especially in Japan, "real-life" observation was lacking. We utilized the national registry of muscular dystrophy in Japan for our retrospective study. We compared the age at loss of ambulation (LOA) between patients in PSL group and those in without-PSL group. Out of 791 patients' in the Remudy DMD/BMD registry from July 2009 to June 2012, 560 were matched with inclusion criteria. Of the 560, all were genetically confirmed DMD patients, 245 (43.8 %) of whom were treated with PSL and 315 (56.2 %) without PSL. There was no difference between the two groups regarding their mutational profile. The age at LOA was significantly greater (11 month on average) in the PSL group than in the without-PSL group (median, 132 vs. 121 months; p = 0.0002). Although strictly controlled clinical trials have shown that corticosteroid therapies achieved a marked improvement in ambulation, discontinuation of the drug due to intolerable side effects led to exclusion of clinical trial participants, which is considered as unavoidable. In our study, patients were not excluded from the PSL group, even if they discontinued the medication shortly after starting it. The results of our study may provide evidence to formulate recommendations and provide a basis for realistic expectations for PSL treatment of DMD patients in Japan, even there are certain limitations due to the retrospectively captured data in the registry.
Figures

References
-
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, Kneile K, Dunn DM, Duval B, Aoyagi A, Hamil C, Mahmoud M, Roush K, Bird L, Rankin C, Lilly H, Street N, Chandrasekar R, Weiss RB. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–313. doi: 10.1002/ana.23528. - DOI - PubMed
-
- Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, King W, Signore L, Pandya S, Florence J, Schierbecker J, Robison J, Kaiser K, Mandel S, Arfken C, Gilder B (1989) Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med 320:1592–1597 - PubMed
-
- Rahman MM, Hannan MA, Mondol BA, Bhoumick NB, Haque A. Prednisolone in Duchenne muscular dystrophy. Bangladesh Med Res Counc Bull. 2001;27:38–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

