Behavioral effects of dopamine receptor inactivation in the caudate-putamen of preweanling rats: role of the D2 receptor
- PMID: 24057816
- PMCID: PMC3946740
- DOI: 10.1007/s00213-013-3280-9
Behavioral effects of dopamine receptor inactivation in the caudate-putamen of preweanling rats: role of the D2 receptor
Abstract
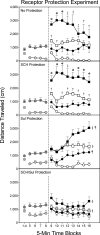
Rationale: Inactivating dopamine (DA) receptors in the caudate-putamen (CPu) attenuates basal and DA agonist-induced behaviors of adult rats while paradoxically increasing the locomotor activity of preweanling rats.
Objective: The purpose of this study was to determine (a) whether D1 or D2 receptor inactivation is responsible for the elevated locomotion shown by preweanling rats and (b) whether DA receptor inactivation produces a general state in which any locomotor-activating drug will cause a potentiated behavioral response.
Methods: Dimethyl sulfoxide (DMSO) or N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) was bilaterally infused into the CPu on postnatal day (PD) 17. In experiment 1, DA receptors were selectively protected from EEDQ-induced alkylation by pretreating rats with D1 and/or D2 antagonists. On PD 18, rats received bilateral microinjections of the DA agonist R(-)-propylnorapomorphine into the dorsal CPu, and locomotor activity was measured for 40 min. In subsequent experiments, the locomotion of DMSO- and EEDQ-pretreated rats was assessed after intraCPu infusions of the selective DA agonists SKF82958 and quinpirole, the partial agonist terguride, or after systemic administration of nonDAergic compounds.
Results: Experiment 1 showed that EEDQ's ability to enhance the locomotor activity of preweanling rats was primarily due to the inactivation of D2 receptors. Consistent with this finding, only drugs that directly or indirectly stimulated D2 receptors produced a potentiated locomotor response in EEDQ-treated rats.
Conclusions: These results show that DA receptor inactivation causes dramatically different behavioral effects in preweanling and adult rats, thus providing additional evidence that the D2 receptor system is not functionally mature by the end of the preweanling period.
Figures





Similar articles
-
Behavioral effects of dopamine receptor inactivation during the adolescent period: age-dependent changes in dorsal striatal D2(High) receptors.Psychopharmacology (Berl). 2014 Apr;231(8):1637-47. doi: 10.1007/s00213-013-3355-7. Epub 2013 Nov 28. Psychopharmacology (Berl). 2014. PMID: 24287603 Free PMC article.
-
Dopamine receptor inactivation in the caudate-putamen differentially affects the behavior of preweanling and adult rats.Neuroscience. 2012 Dec 13;226:427-40. doi: 10.1016/j.neuroscience.2012.09.030. Epub 2012 Sep 19. Neuroscience. 2012. PMID: 23000622 Free PMC article.
-
Importance of D1 and D2 receptors in the dorsal caudate-putamen for the locomotor activity and stereotyped behaviors of preweanling rats.Neuroscience. 2011 Jun 2;183:121-33. doi: 10.1016/j.neuroscience.2011.03.037. Epub 2011 Apr 2. Neuroscience. 2011. PMID: 21443930 Free PMC article.
-
Behavioral effects of selective and nonselective dopamine agonists on young rats after irreversible antagonism of D1 and/or D2 receptors.Psychopharmacology (Berl). 1993;111(2):225-32. doi: 10.1007/BF02245528. Psychopharmacology (Berl). 1993. PMID: 7870957
-
Interactions between D1 and D2 dopamine receptor family agonists and antagonists: the effects of chronic exposure on behavior and receptor binding in rats and their clinical implications.J Neural Transm (Vienna). 1997;104(4-5):341-62. doi: 10.1007/BF01277656. J Neural Transm (Vienna). 1997. PMID: 9295170 Review.
Cited by
-
Medial prefrontal cortex diclofenac-induced antinociception is mediated through GPR55, cannabinoid CB1, and mu-opioid receptors of this area and periaqueductal gray.Naunyn Schmiedebergs Arch Pharmacol. 2020 Mar;393(3):371-379. doi: 10.1007/s00210-019-01735-x. Epub 2019 Oct 22. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31641818
-
Behavioral effects of dopamine receptor inactivation during the adolescent period: age-dependent changes in dorsal striatal D2(High) receptors.Psychopharmacology (Berl). 2014 Apr;231(8):1637-47. doi: 10.1007/s00213-013-3355-7. Epub 2013 Nov 28. Psychopharmacology (Berl). 2014. PMID: 24287603 Free PMC article.
-
Dopamine D2 Receptor Supersensitivity as a Spectrum of Neurotoxicity and Status in Psychiatric Disorders.J Pharmacol Exp Ther. 2018 Sep;366(3):519-526. doi: 10.1124/jpet.118.247981. Epub 2018 Jun 19. J Pharmacol Exp Ther. 2018. PMID: 29921706 Free PMC article. Review.
-
Age-dependent effects of dopamine receptor inactivation on cocaine-induced behaviors in male rats: Evidence of dorsal striatal D2 receptor supersensitivity.J Neurosci Res. 2019 Dec;97(12):1546-1558. doi: 10.1002/jnr.24491. Epub 2019 Jul 15. J Neurosci Res. 2019. PMID: 31304635 Free PMC article.
-
Age-dependent changes in cocaine sensitivity across early ontogeny in male and female rats: possible role of dorsal striatal D2(High) receptors.Psychopharmacology (Berl). 2015 Jul;232(13):2287-301. doi: 10.1007/s00213-014-3860-3. Epub 2015 Jan 16. Psychopharmacology (Berl). 2015. PMID: 25589144 Free PMC article.
References
-
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. - PubMed
-
- Arnt J, Hyttel J. Postsynaptic dopamine agonistic effects of 3-PPP enantiomers revealed by bilateral 6-hydroxy-dopamine lesions and by chronic reserpine treatment in rats. J Neural Transm. 1984;60:205–223. - PubMed
-
- Arnt J, Hyttel J. Dopamine D-2 agonists with high and low efficacies: differentiation by behavioural techniques. J Neural Transm. 1990;80:33–50. - PubMed
-
- Arnt J, Hyttel J, Meier E. Inactivation of dopamine D-1 or D-2 receptors differentially inhibits stereotypies induced by dopamine agonists in rats. Eur J Pharmacol. 1988;155:37–47. - PubMed
-
- Bordi F, Carr KD, Meller E. Stereotypies elicited by injection of N-propylnorapomorphine into striatal subregions and nucleus accumbens. Brain Res. 1989;489:205–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

