Topiramate for smoking cessation: a randomized, placebo-controlled pilot study
- PMID: 24057996
- PMCID: PMC3920334
- DOI: 10.1093/ntr/ntt141
Topiramate for smoking cessation: a randomized, placebo-controlled pilot study
Abstract
Introduction: Topiramate (TOP) blocks glutamate receptors and facilitates GABA (γ-aminobutyric acid) neurotransmission, effects that may facilitate smoking cessation. We compared the effects of behavioral counseling combined with (a) TOP, (b) TOP/nicotine patch (TOP/NIC), or (c) placebo (PLC) for smoking cessation.
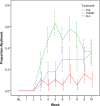
Methods: We conducted a 10-week randomized trial in which subjects and research personnel were blinded to TOP versus PLC but not to the TOP/NIC patch condition. In groups receiving TOP, the medication dosage was titrated gradually up to 200 mg/day. The smoking quit date (QD) was scheduled after 2 weeks of medication treatment. NIC (21 mg) was started on the QD in subjects randomized to the TOP/NIC condition. The main outcome measure was the end-of-treatment, 4-week continuous abstinence rate (CAR; biochemically confirmed).
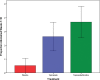
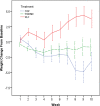
Results: Fifty-seven subjects were randomized to treatment. The 4-week CAR was 1 of 19 (5%) in the PLC group, 5 of 19 (26%) in the TOP group, and 7 of 19 (37%) in the TOP/NIC group (p = .056). Pairwise comparisons showed a difference between TOP/NIC and PLC (p = .042) and a nonsignificant difference between TOP and PLC (p = .18). The PLC group gained 0.37 lb/week, the TOP group lost 0.41 lb/week, and the TOP/NIC group lost 0.07 lb/week (p = .004). Pairwise comparisons showed a difference between TOP and PLC (p < .001) and between TOP/NIC and PLC groups (p = .035). Paresthesia was more frequent in subjects on TOP than PLC (p = .011).
Conclusions: TOP, alone or in combination with the NIC, resulted in a numerically higher quit rate than PLC and decreased weight. A larger, PLC-controlled trial is needed to confirm these findings.
Figures
References
-
- Anthenelli R. M., Blom T. J., McElroy S. L., Keck P. E., Jr. (2008). Preliminary evidence for gender-specific effects of topiramate as a potential aid to smoking cessation. Addiction, 103, 687–694. ADD2148 10.1111/j.1360-0443.2008.02148.x - PubMed
-
- Bodalia P. N., Grosso A. M., Sofat R., Macallister R. J., Smeeth L., Dhillon S. … Hingorani A. D. (2013). Comparative Efficacy and Tolerability of Antiepileptic Drugs for Refractory Focal Epilepsy Systematic Review and Network Meta-Analysis reveals the need for long-term comparator trials. British Journal of Clinical Pharmacology. 10.1111/bcp.12083 - PMC - PubMed
-
- Fiore M. C., Jaén C. R., Baker T. B., Bailey W. C., Benowitz N. L., Curry S. J. … Wewers M. (2008). Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service. 10.1016/j.amepre.2008.04.009
-
- Fontenelle L. F., Oostermeijer S., Harrison B. J., Pantelis C., Yücel M. (2011). Obsessive-compulsive disorder, impulse control disorders and drug addiction: Common features and potential treatments. Drugs, 71, 827–840. 10.2165/11591790-000000000-00000 2 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

