Lung epithelial branching program antagonizes alveolar differentiation
- PMID: 24058167
- PMCID: PMC3831485
- DOI: 10.1073/pnas.1311760110
Lung epithelial branching program antagonizes alveolar differentiation
Abstract
Mammalian organs, including the lung and kidney, often adopt a branched structure to achieve high efficiency and capacity of their physiological functions. Formation of a functional lung requires two developmental processes: branching morphogenesis, which builds a tree-like tubular network, and alveolar differentiation, which generates specialized epithelial cells for gas exchange. Much progress has been made to understand each of the two processes individually; however, it is not clear whether the two processes are coordinated and how they are deployed at the correct time and location. Here we show that an epithelial branching morphogenesis program antagonizes alveolar differentiation in the mouse lung. We find a negative correlation between branching morphogenesis and alveolar differentiation temporally, spatially, and evolutionarily. Gain-of-function experiments show that hyperactive small GTPase Kras expands the branching program and also suppresses molecular and cellular differentiation of alveolar cells. Loss-of-function experiments show that SRY-box containing gene 9 (Sox9) functions downstream of Fibroblast growth factor (Fgf)/Kras to promote branching and also suppresses premature initiation of alveolar differentiation. We thus propose that lung epithelial progenitors continuously balance between branching morphogenesis and alveolar differentiation, and such a balance is mediated by dual-function regulators, including Kras and Sox9. The resulting temporal delay of differentiation by the branching program may provide new insights to lung immaturity in preterm neonates and the increase in organ complexity during evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


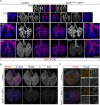
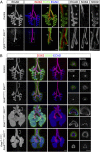

Comment in
-
Balancing the developmental niches within the lung.Proc Natl Acad Sci U S A. 2013 Nov 5;110(45):18029-30. doi: 10.1073/pnas.1317795110. Epub 2013 Oct 24. Proc Natl Acad Sci U S A. 2013. PMID: 24158480 Free PMC article. No abstract available.
References
-
- De Moerlooze L, et al. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127(3):483–492. - PubMed
-
- Sekine K, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21(1):138–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

