Differential joint-specific corticospinal tract projections within the cervical enlargement
- PMID: 24058570
- PMCID: PMC3776849
- DOI: 10.1371/journal.pone.0074454
Differential joint-specific corticospinal tract projections within the cervical enlargement
Abstract
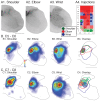
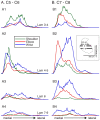
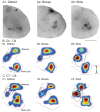
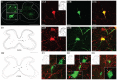
The motor cortex represents muscle and joint control and projects to spinal cord interneurons and-in many primates, including humans-motoneurons, via the corticospinal tract (CST). To examine these spinal CST anatomical mechanisms, we determined if motor cortex sites controlling individual forelimb joints project differentially to distinct cervical spinal cord territories, defined regionally and by the locations of putative last-order interneurons that were transneuronally labeled by intramuscular injection of pseudorabies virus. Motor cortex joint-specific sites were identified using intracortical-microstimulation. CST segmental termination fields from joint-specific sites, determined using anterograde tracers, comprised a high density core of terminations that was consistent between animals and a surrounding lower density projection that was more variable. Core terminations from shoulder, elbow, and wrist control sites overlapped in the medial dorsal horn and intermediate zone at C5/C6 but were separated at C7/C8. Shoulder sites preferentially terminated dorsally, in the dorsal horn; wrist/digit sites, more ventrally in the intermediate zone; and elbow sites, medially in the dorsal horn and intermediate zone. Pseudorabies virus injected in shoulder, elbow, or wrist muscles labeled overlapping populations of predominantly muscle-specific putative premotor interneurons, at a survival time for disynaptic transfer from muscle. At C5/C6, CST core projections from all joint zones were located medial to regions of densely labeled last-order interneurons, irrespective of injected muscle. At C7/C8 wrist CST core projections overlapped the densest interneuron territory, which was located in the lateral intermediate zone. In contrast, elbow CST core projections were located medial to the densest interneuron territories, and shoulder CST core projections were located dorsally and only partially overlapped the densest interneuron territory. Our findings show a surprising fractionation of CST terminations in the caudal cervical enlargement that may be organized to engage different spinal premotor circuits for distal and proximal joint control.
Conflict of interest statement
Figures



References
-
- Porter R, Lemon R (1993) Corticospinal function and voluntary movement. Oxford: Oxford Science. 428 p.
-
- Asanuma H, Arnold A, Zarzecki P (1976) Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp Brain Res 26: 443–461. - PubMed
-
- Graziano MS, Taylor CS, Moore T (2002) Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851. - PubMed
-
- Churchland MM, Shenoy KV (2007) Temporal complexity and heterogeneity of single-neuron activity in premotor and motor cortex. Journal of neurophysiology 97: 4235–4257. - PubMed
-
- Kuypers HGJM (1981) Anatomy of the descending pathways. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, Neurophysiology, Vol II. Bethesda,MD: Am. Physiol.Soc. 597–666.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

