Aging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liver
- PMID: 24058615
- PMCID: PMC3772924
- DOI: 10.1371/journal.pone.0074644
Aging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liver
Abstract
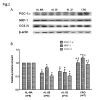
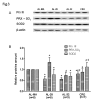
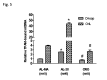
Aging affects mitochondria in a tissue-specific manner. Calorie restriction (CR) is, so far, the only intervention able to delay or prevent the onset of several age-related changes also in mitochondria. Using livers from middle age (18-month-old), 28-month-old and 32-month-old ad libitum-fed and 28-month-old calorie-restricted rats we found an age-related decrease in mitochondrial DNA (mtDNA) content and mitochondrial transcription factor A (TFAM) amount, fully prevented by CR. We revealed also an age-related decrease, completely prevented by CR, for the proteins PGC-1α NRF-1 and cytochrome c oxidase subunit IV, supporting the efficiency of CR to forestall the age-related decrease in mitochondrial biogenesis. Furthermore, CR counteracted the age-related increase in oxidative damage to proteins, represented by the increased amount of oxidized peroxiredoxins (PRX-SO3) in the ad libitum-fed animals. An unexpected age-related decrease in the mitochondrial proteins peroxiredoxin III (Prx III) and superoxide dismutase 2 (SOD2), usually induced by increased ROS and involved in mitochondrial biogenesis, suggested a prevailing relevance of the age-reduced mitochondrial biogenesis above the induction by ROS in the regulation of expression of these genes with aging. The partial prevention of the decrease in Prx III and SOD2 proteins by CR also supported the preservation of mitochondrial biogenesis in the anti-aging action of CR. To investigate further the age- and CR-related effects on mitochondrial biogenesis we analyzed the in vivo binding of TFAM to specific mtDNA regions and demonstrated a marked increase in the TFAM-bound amounts of mtDNA at both origins of replication with aging, fully prevented by CR. A novel, positive correlation between the paired amounts of TFAM-bound mtDNA at these sub-regions was found in the joined middle age ad libitum-fed and 28-month-old calorie-restricted groups, but not in the 28-month-old ad libitum-fed counterpart suggesting a quite different modulation of TFAM binding at both origins of replication in aging and CR.
Conflict of interest statement
Figures



References
-
- Wallace DC (1992) Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science 256: 628-632. doi:10.1126/science.1533953. PubMed: 1533953. - DOI - PubMed
-
- Huang JH, Hood DA (2009) Age-associated mitochondrial dysfunction in skeletal muscle: Contributing factors and suggestions for long-term interventions. IUBMB Life 61: 201-214. doi:10.1002/iub.164. PubMed: 19243006. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

