Ancient neuromodulation by vasopressin/oxytocin-related peptides
- PMID: 24058873
- PMCID: PMC3704447
- DOI: 10.4161/worm.24246
Ancient neuromodulation by vasopressin/oxytocin-related peptides
Abstract
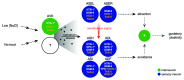
Neuropeptidergic signaling is widely adopted by animals for the regulation of physiology and behavior in a rapidly changing environment. The vasopressin/oxytocin neuropeptide family originates from an ancestral peptide precursor in the antecedent of protostomian and deuterostomian animals. In vertebrates, vasopressin and oxytocin have both hormonal effects on peripheral target tissues, such as in the regulation of reproduction and water balance, and neuromodulatory actions in the central nervous system controlling social behavior and cognition. The recent identification of vasopressin/oxytocin-related signaling in C. elegans reveals that this peptidergic system is widespread among nematodes. Genetic analysis of the C. elegans nematocin system denotes vasopressin/oxytocin-like peptides as ancient neuromodulators of neuronal circuits involved in reproductive behavior and associative learning, whereas former invertebrate studies focused on conserved peripheral actions of this peptide family. Nematocin provides neuromodulatory input into the gustatory plasticity circuit as well as into distinct male mating circuits to generate a coherent mating behavior. Molecular interactions are comparable to those underlying vasopressin- and oxytocin-mediated effects in the mammalian brain. Understanding how the vasopressin/oxytocin family fine-tunes neuronal circuits for social behavior, learning and memory poses a major challenge. Functional conservation of these effects in nematodes and most likely in other invertebrates enables the development of future models to help answering this question.
Keywords: C. elegans; learning; nematocin; neuromodulation; neuropeptide; oxytocin; reproductive behavior; vasopressin.
Figures

References
-
- Du Vigneaud V, Ressler C, Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953;205:949–57. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
