FCET2EC (From controlled experimental trial to = 2 everyday communication): How effective is intensive integrative therapy for stroke-induced chronic aphasia under routine clinical conditions? A study protocol for a randomized controlled trial
- PMID: 24059983
- PMCID: PMC3850954
- DOI: 10.1186/1745-6215-14-308
FCET2EC (From controlled experimental trial to = 2 everyday communication): How effective is intensive integrative therapy for stroke-induced chronic aphasia under routine clinical conditions? A study protocol for a randomized controlled trial
Abstract
Background: Therapy guidelines recommend speech and language therapy (SLT) as the "gold standard" for aphasia treatment. Treatment intensity (i.e., ≥5 hours of SLT per week) is a key predictor of SLT outcome. The scientific evidence to support the efficacy of SLT is unsatisfactory to date given the lack of randomized controlled trials (RCT), particularly with respect to chronic aphasia (lasting for >6 months after initial stroke). This randomized waiting list-controlled multi-centre trial examines whether intensive integrative language therapy provided in routine in- and outpatient clinical settings is effective in improving everyday communication in chronic post-stroke aphasia.
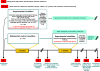
Methods/design: Participants are men and women aged 18 to 70 years, at least 6 months post an ischemic or haemorrhagic stroke resulting in persisting language impairment (i.e., chronic aphasia); 220 patients will be screened for participation, with the goal of including at least 126 patients during the 26-month recruitment period. Basic language production and comprehension abilities need to be preserved (as assessed by the Aachen Aphasia Test).Therapy consists of language-systematic and communicative-pragmatic exercises for at least 2 hours/day and at least 10 hours/week, plus at least 1 hour self-administered training per day, for at least three weeks. Contents of therapy are adapted to patients' individual impairment profiles.Prior to and immediately following the therapy/waiting period, patients' individual language abilities are assessed via primary and secondary outcome measures. The primary (blinded) outcome measure is the A-scale (informational content, or 'understandability', of the message) of the Amsterdam-Nijmegen Everyday Language Test (ANELT), a standardized measure of functional communication ability. Secondary (unblinded) outcome measures are language-systematic and communicative-pragmatic language screenings and questionnaires assessing life quality as viewed by the patient as well as a relative.The primary analysis tests for differences between the therapy group and an untreated (waiting list) control group with respect to pre- versus post 3-week-therapy (or waiting period, respectively) scores on the ANELT A-scale. Statistical between-group comparisons of primary and secondary outcome measures will be conducted in intention-to-treat analyses.Long-term stability of treatment effects will be assessed six months post intensive SLT (primary and secondary endpoints).
Trial registration: Registered in ClinicalTrials.gov with the Identifier NCT01540383.
References
-
- Cherney LR, Patterson JP, Raymer A, Frymark T, Schooling T. Evidence-based systematic review: effects of intensity of treatment and constraint-induced language therapy for individuals with stroke-induced aphasia. J Speech Lang Hear Res. 2008;51:1282–1299. doi: 10.1044/1092-4388(2008/07-0206). An updated version of the original review dated October 2010 is available at http://www.asha.org/uploadedFiles/EBSR-Updated-CILT.pdf. - DOI - PubMed
-
- Salter K, Teasell R, Bhogal S, Zettler L, Foley N. Aphasia. Evidence-Based Review of Stroke Rehabilitation. 15. 2012. http://www.ebrsr.com/papers_details.php?3.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical