Proliferation and estrogen signaling can distinguish patients at risk for early versus late relapse among estrogen receptor positive breast cancers
- PMID: 24060333
- PMCID: PMC3978752
- DOI: 10.1186/bcr3481
Proliferation and estrogen signaling can distinguish patients at risk for early versus late relapse among estrogen receptor positive breast cancers
Abstract
Introduction: We examined if a combination of proliferation markers and estrogen receptor (ER) activity could predict early versus late relapses in ER-positive breast cancer and inform the choice and length of adjuvant endocrine therapy.
Methods: Baseline affymetrix gene-expression profiles from ER-positive patients who received no systemic therapy (n = 559), adjuvant tamoxifen for 5 years (cohort-1: n = 683, cohort-2: n = 282) and from 58 patients treated with neoadjuvant letrozole for 3 months (gene-expression available at baseline, 14 and 90 days) were analyzed. A proliferation score based on the expression of mitotic kinases (MKS) and an ER-related score (ERS) adopted from Oncotype DX® were calculated. The same analysis was performed using the Genomic Grade Index as proliferation marker and the luminal gene score from the PAM50 classifier as measure of estrogen-related genes. Median values were used to define low and high marker groups and four combinations were created. Relapses were grouped into time cohorts of 0-2.5, 0-5, 5-10 years.
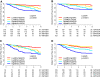
Results: In the overall 10 years period, the proportional hazards assumption was violated for several biomarker groups indicating time-dependent effects. In tamoxifen-treated patients Low-MKS/Low-ERS cancers had continuously increasing risk of relapse that was higher after 5 years than Low-MKS/High-ERS cancers [0 to 10 year, HR 3.36; p = 0.013]. High-MKS/High-ERS cancers had low risk of early relapse [0-2.5 years HR 0.13; p = 0.0006], but high risk of late relapse which was higher than in the High-MKS/Low-ERS group [after 5 years HR 3.86; p = 0.007]. The High-MKS/Low-ERS subset had most of the early relapses [0 to 2.5 years, HR 6.53; p < 0.0001] especially in node negative tumors and showed minimal response to neoadjuvant letrozole. These findings were qualitatively confirmed in a smaller independent cohort of tamoxifen-treated patients. Using different biomarkers provided similar results.
Conclusions: Early relapses are highest in highly proliferative/low-ERS cancers, in particular in node negative tumors. Relapses occurring after 5 years of adjuvant tamoxifen are highest among the highly-proliferative/high-ERS tumors although their risk of recurrence is modest in the first 5 years on tamoxifen. These tumors could be the best candidates for extended endocrine therapy.
Figures




References
-
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Alencar VHM, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou M-F, Inbar M, Khaled H, Kielanowska J, Kwan W-H, Mathew BS, Müller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Rajan B. et al.Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;15:805–816. doi: 10.1016/S0140-6736(12)61963-1. - DOI - PMC - PubMed
-
- Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;15:55–65. doi: 10.1016/S1470-2045(09)70314-6. - DOI - PMC - PubMed
-
- Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, Davies SR, Snider J, Stijleman IJ, Reed J, Cheang MC, Mardis ER, Perou CM, Bernard PS, Ellis MJ. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;15:5222–5232. doi: 10.1158/1078-0432.CCR-10-1282. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

