Human progenitor cell recruitment via SDF-1α coacervate-laden PGS vascular grafts
- PMID: 24060423
- PMCID: PMC3882008
- DOI: 10.1016/j.biomaterials.2013.08.082
Human progenitor cell recruitment via SDF-1α coacervate-laden PGS vascular grafts
Abstract
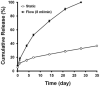
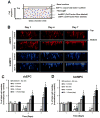
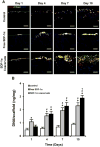
Host cell recruitment is crucial for vascular graft remodeling and integration into the native blood vessel; it is especially important for cell-free strategies which rely on host remodeling. Controlled release of growth factors from vascular grafts may enhance host cell recruitment. Stromal cell-derived factor (SDF)-1α has been shown to induce host progenitor cell migration and recruitment; however, its potential in regenerative therapies is often limited due to its short half-life in vivo. This report describes a coacervate drug delivery system for enhancing progenitor cell recruitment into an elastomeric vascular graft by conferring protection of SDF-1α. Heparin and a synthetic polycation are used to form a coacervate, which is incorporated into poly(glycerol sebacate) (PGS) scaffolds. In addition to protecting SDF-1α, the coacervate facilitates uniform scaffold coating. Coacervate-laden scaffolds have high SDF-1α loading efficiency and provide sustained release under static and physiologically-relevant flow conditions with minimal initial burst release. In vitro assays showed that coacervate-laden scaffolds enhance migration and infiltration of human endothelial and mesenchymal progenitor cells by maintaining a stable SDF-1α gradient. These results suggest that SDF-1α coacervate-laden scaffolds show great promise for in situ vascular regeneration.
Keywords: Coacervate; Human progenitor cells; Poly(glycerol sebacate); Polycation; Stromal cell-derived factor (SDF)-1α; Tissue engineering.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
-
- Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. - PubMed
-
- Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284:489–93. - PubMed
-
- L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. Faseb J. 1998;12:47–56. - PubMed
-
- Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

