CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations
- PMID: 24060820
- PMCID: PMC3904554
- DOI: 10.1038/clpt.2013.186
CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations
Abstract
The International Tamoxifen Pharmacogenomics Consortium was established to address the controversy regarding cytochrome P450 2D6 (CYP2D6) status and clinical outcomes in tamoxifen therapy. We performed a meta-analysis on data from 4,973 tamoxifen-treated patients (12 globally distributed sites). Using strict eligibility requirements (postmenopausal women with estrogen receptor-positive breast cancer, receiving 20 mg/day tamoxifen for 5 years, criterion 1); CYP2D6 poor metabolizer status was associated with poorer invasive disease-free survival (IDFS: hazard ratio = 1.25; 95% confidence interval = 1.06, 1.47; P = 0.009). However, CYP2D6 status was not statistically significant when tamoxifen duration, menopausal status, and annual follow-up were not specified (criterion 2, n = 2,443; P = 0.25) or when no exclusions were applied (criterion 3, n = 4,935; P = 0.38). Although CYP2D6 is a strong predictor of IDFS using strict inclusion criteria, because the results are not robust to inclusion criteria (these were not defined a priori), prospective studies are necessary to fully establish the value of CYP2D6 genotyping in tamoxifen therapy.
Figures

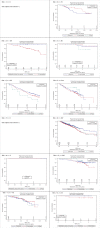
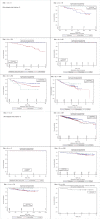
Comment in
-
CYP2D6 genotype and adjuvant tamoxifen.Clin Pharmacol Ther. 2014 Aug;96(2):138-40. doi: 10.1038/clpt.2014.96. Clin Pharmacol Ther. 2014. PMID: 25056391 No abstract available.
References
-
- Jordan V.C. Tamoxifen: a most unlikely pioneering medicine. Nat. Rev. Drug Discov. 2003;2:205–213. - PubMed
-
- Johnson M.D., et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res. Treat. 2004;85:151–159. - PubMed
-
- Stearns V., et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Natl. Cancer Inst. 2003;95:1758–1764. - PubMed
-
- Goetz M.P., Berry D.A., Klein T.E. 31136 Findings from the International Tamoxifen Pharmacogenomics Consortium. Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, Texas. 9–13 December. 2009. Abstract 33
Publication types
MeSH terms
Substances
Grants and funding
- CA-60274/CA/NCI NIH HHS/United States
- CA35103/CA/NCI NIH HHS/United States
- CA-35195/CA/NCI NIH HHS/United States
- U10 CA035448/CA/NCI NIH HHS/United States
- U10 CA035195/CA/NCI NIH HHS/United States
- CA-25224/CA/NCI NIH HHS/United States
- R01 CA132780/CA/NCI NIH HHS/United States
- T32 GM007546/GM/NIGMS NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- CA-35415/CA/NCI NIH HHS/United States
- U19 GM061388/GM/NIGMS NIH HHS/United States
- CA-51008/CA/NCI NIH HHS/United States
- R24 GM061374/GM/NIGMS NIH HHS/United States
- U19 GM61388/GM/NIGMS NIH HHS/United States
- CA-58207/CA/NCI NIH HHS/United States
- U10 CA037417/CA/NCI NIH HHS/United States
- U10 CA035269/CA/NCI NIH HHS/United States
- CA-37404/CA/NCI NIH HHS/United States
- CA-116201/CA/NCI NIH HHS/United States
- CA-52352/CA/NCI NIH HHS/United States
- U10 CA037404/CA/NCI NIH HHS/United States
- R01 CA133049/CA/NCI NIH HHS/United States
- P30 CA051008/CA/NCI NIH HHS/United States
- P50 CA058207/CA/NCI NIH HHS/United States
- U10 CA035272/CA/NCI NIH HHS/United States
- P50 CA116201/CA/NCI NIH HHS/United States
- CA-35113/CA/NCI NIH HHS/United States
- U10 CA035101/CA/NCI NIH HHS/United States
- U10 CA035415/CA/NCI NIH HHS/United States
- U10 CA063849/CA/NCI NIH HHS/United States
- CA-37417/CA/NCI NIH HHS/United States
- CA-35272/CA/NCI NIH HHS/United States
- CA-35269/CA/NCI NIH HHS/United States
- CA-35448/CA/NCI NIH HHS/United States
- 1R01CA133049-01/CA/NCI NIH HHS/United States
- R24 GM61374/GM/NIGMS NIH HHS/United States
- CA-35101/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- CA-15083/CA/NCI NIH HHS/United States
- CRUK_/Cancer Research UK/United Kingdom
- CA-63849/CA/NCI NIH HHS/United States
- CA051008/CA/NCI NIH HHS/United States
- N01 CA015083/CA/NCI NIH HHS/United States
- U10 CA035103/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

