Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction
- PMID: 24061088
- PMCID: PMC3907064
- DOI: 10.1161/CIRCULATIONAHA.113.002863
Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction
Abstract
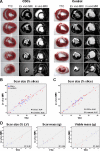
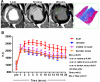
Background: Magnetic resonance imaging (MRI) in the CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction (CADUCEUS) trial revealed that cardiosphere-derived cells (CDCs) decrease scar size and increase viable myocardium after myocardial infarction (MI), but MRI has not been validated as an index of regeneration after cell therapy. We tested the validity of contrast-enhanced MRI in quantifying scarred and viable myocardium after cell therapy in a porcine model of convalescent MI.
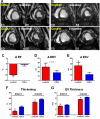
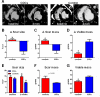
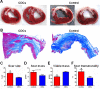
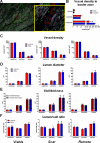
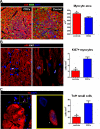
Methods and results: Yucatan minipigs underwent induction of MI and 2-3 weeks later were randomized to receive intracoronary infusion of 12.5×10(6) mismatched allogeneic CDCs or vehicle. Allogeneic CDCs induced mild local mononuclear infiltration but no systemic immunogenicity. MRI revealed that allogeneic CDCs attenuated remodeling, improved global and regional function, decreased scar size, and increased viable myocardium compared with placebo 2 months post-treatment. Extensive histological analysis validated quantitatively the MRI measurements of scar size, scar mass, and viable mass. CDCs neither altered gadolinium contrast myocardial kinetics nor induced changes in vascular density or architecture in viable and scarred myocardium. Histology demonstrated that CDCs lead to cardiomyocyte hyperplasia in the border zone, consistent with the observed stimulation of endogenous regenerative mechanisms (cardiomyocyte cycling, upregulation of endogenous progenitors, angiogenesis).
Conclusions: Contrast-enhanced MRI accurately measures scarred and viable myocardium after cell therapy in a porcine model of convalescent MI. MRI represents a useful tool for assessing dynamic changes in the infarct and monitoring regenerative efficacy.
Keywords: adult stem cells; allogeneic transplantation; cell transplantation; magnetic resonance imaging; myocardial infarction; regeneration.
Figures

References
-
- Dauwe DF, Janssens SP. Stem cell therapy for the treatment of myocardial infarction. Curr Pharm Des. 2011;17:3328–3340. - PubMed
-
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. - PMC - PubMed
-
- Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11 Suppl 1):S54–64. - PMC - PubMed
-
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

