Downregulation of PHLPP expression contributes to hypoxia-induced resistance to chemotherapy in colon cancer cells
- PMID: 24061475
- PMCID: PMC3838188
- DOI: 10.1128/MCB.00695-13
Downregulation of PHLPP expression contributes to hypoxia-induced resistance to chemotherapy in colon cancer cells
Abstract
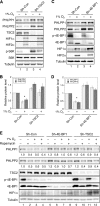
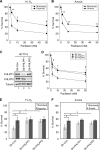
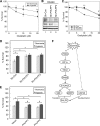
Hypoxia is a feature of solid tumors. Most tumors are at least partially hypoxic. This hypoxic environment plays a critical role in promoting resistance to anticancer drugs. PHLPP, a novel family of Ser/Thr protein phosphatases, functions as a tumor suppressor in colon cancers. Here, we show that the expression of both PHLPP isoforms is negatively regulated by hypoxia/anoxia in colon cancer cells. Interestingly, a hypoxia-induced decrease of PHLPP expression is attenuated by knocking down HIF1α but not HIF2α. Whereas the mRNA levels of PHLPP are not significantly altered by oxygen deprivation, the reduction of PHLPP expression is caused by decreased protein translation downstream of mTOR and increased degradation. Specifically, hypoxia-induced downregulation of PHLPP is partially rescued in TSC2 or 4E-BP1 knockdown cells as the result of elevated mTOR activity and protein synthesis. Moreover, oxygen deprivation destabilizes PHLPP protein by decreasing the expression of USP46, a deubiquitinase of PHLPP. Functionally, downregulation of PHLPP contributes to hypoxia-induced chemoresistance in colon cancer cells. Taken together, we have identified hypoxia as a novel mechanism by which PHLPP is downregulated in colon cancer, and the expression of PHLPP may serve as a biomarker for better understanding of chemoresistance in cancer treatment.
Figures




Similar articles
-
mTOR-dependent regulation of PHLPP expression controls the rapamycin sensitivity in cancer cells.J Biol Chem. 2011 Feb 25;286(8):6510-20. doi: 10.1074/jbc.M110.183087. Epub 2010 Dec 22. J Biol Chem. 2011. PMID: 21177869 Free PMC article.
-
The deubiquitination enzyme USP46 functions as a tumor suppressor by controlling PHLPP-dependent attenuation of Akt signaling in colon cancer.Oncogene. 2013 Jan 24;32(4):471-8. doi: 10.1038/onc.2012.66. Epub 2012 Mar 5. Oncogene. 2013. PMID: 22391563 Free PMC article.
-
Downregulation of PHLPP induced by endoplasmic reticulum stress promotes eIF2α phosphorylation and chemoresistance in colon cancer.Cell Death Dis. 2021 Oct 18;12(11):960. doi: 10.1038/s41419-021-04251-0. Cell Death Dis. 2021. PMID: 34663797 Free PMC article.
-
Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways.Drug Resist Updat. 2011 Jun;14(3):191-201. doi: 10.1016/j.drup.2011.03.001. Epub 2011 Apr 3. Drug Resist Updat. 2011. PMID: 21466972 Review.
-
PHLPPing through history: a decade in the life of PHLPP phosphatases.Biochem Soc Trans. 2016 Dec 15;44(6):1675-1682. doi: 10.1042/BST20160170. Biochem Soc Trans. 2016. PMID: 27913677 Free PMC article. Review.
Cited by
-
Oncogenic Ras differentially regulates metabolism and anoikis in extracellular matrix-detached cells.Cell Death Differ. 2016 Aug;23(8):1271-82. doi: 10.1038/cdd.2016.15. Epub 2016 Feb 26. Cell Death Differ. 2016. PMID: 26915296 Free PMC article.
-
The role of hypoxia-inducible factor-2 in digestive system cancers.Cell Death Dis. 2015 Jan 15;6(1):e1600. doi: 10.1038/cddis.2014.565. Cell Death Dis. 2015. PMID: 25590810 Free PMC article. Review.
-
Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape.J Hematol Oncol. 2022 Jun 3;15(1):77. doi: 10.1186/s13045-022-01292-6. J Hematol Oncol. 2022. PMID: 35659268 Free PMC article. Review.
-
Comprehensive analysis of the role of ubiquitin-specific peptidases in colorectal cancer: A systematic review.World J Gastrointest Oncol. 2024 Jan 15;16(1):197-213. doi: 10.4251/wjgo.v16.i1.197. World J Gastrointest Oncol. 2024. PMID: 38292842 Free PMC article.
-
Pleckstrin Homology (PH) Domain Leucine-rich Repeat Protein Phosphatase Controls Cell Polarity by Negatively Regulating the Activity of Atypical Protein Kinase C.J Biol Chem. 2016 Nov 25;291(48):25167-25178. doi: 10.1074/jbc.M116.740639. Epub 2016 Oct 19. J Biol Chem. 2016. PMID: 27760826 Free PMC article.
References
-
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. 2001. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472 - PubMed
-
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
