Biomedically relevant circuit-design strategies in mammalian synthetic biology
- PMID: 24061539
- PMCID: PMC3792348
- DOI: 10.1038/msb.2013.48
Biomedically relevant circuit-design strategies in mammalian synthetic biology
Abstract
The development and progress in synthetic biology has been remarkable. Although still in its infancy, synthetic biology has achieved much during the past decade. Improvements in genetic circuit design have increased the potential for clinical applicability of synthetic biology research. What began as simple transcriptional gene switches has rapidly developed into a variety of complex regulatory circuits based on the transcriptional, translational and post-translational regulation. Instead of compounds with potential pharmacologic side effects, the inducer molecules now used are metabolites of the human body and even members of native cell signaling pathways. In this review, we address recent progress in mammalian synthetic biology circuit design and focus on how novel designs push synthetic biology toward clinical implementation. Groundbreaking research on the implementation of optogenetics and intercellular communications is addressed, as particularly optogenetics provides unprecedented opportunities for clinical application. Along with an increase in synthetic network complexity, multicellular systems are now being used to provide a platform for next-generation circuit design.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
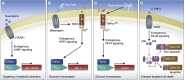
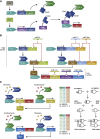
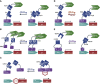

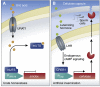

References
-
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K (2009) Temporally precise in vivo control of intracellular signalling. Nature 458: 1025–1029 - PubMed
-
- Anderson JC, Clarke EJ, Arkin AP, Voigt CA (2006) Environmentally controlled invasion of cancer cells by engineered bacteria. J Mol Biol 355: 619–627 - PubMed
-
- Arrenberg AB, Stainier DY, Baier H, Huisken J (2010) Optogenetic control of cardiac function. Science 330: 971–974 - PubMed
-
- Auslander S, Auslander D, Muller M, Wieland M, Fussenegger M (2012a) Programmable single-cell mammalian biocomputers. Nature 487: 123–127 - PubMed
-
- Auslander S, Fussenegger M (2013) From gene switches to mammalian designer cells: present and future prospects. Trends Biotechnol 31: 155–168 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

