DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA
- PMID: 24062211
- PMCID: PMC3839302
- DOI: 10.1007/s10549-013-2703-y
DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA
Abstract
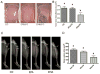
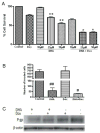
Breast cancer patients often develop bone metastasis evidenced by osteolytic lesions, leading to severe pain and bone fracture. Attenuation of breast cancer metastasis to bone and associated osteolysis by fish oil, rich in EPA and DHA, has been demonstrated previously. However, it was not known whether EPA and DHA differentially or similarly affect breast cancer bone metastasis and associated osteolysis. In vitro culture of parental and luciferase gene encoded MDA-MB-231 human breast cancer cell lines treated with EPA and DHA revealed that DHA inhibits proliferation and invasion of breast cancer cells more potently than EPA. Intra-cardiac injection of parental and luciferase gene encoded MDA-MB-231 cells to athymic NCr nu/nu mice demonstrated that DHA-treated mice had significantly less breast cancer cell burden in bone, and also significantly less osteolytic lesions than EPA-treated mice. In vivo cell migration assay as measured by luciferase intensity revealed that DHA attenuated cell migration specifically to the bone. Moreover, the DHA-treated group showed reduced levels of CD44 and TRAP positive area in bone compared to EPA-treated group. Breast cancer cell burden and osteolytic lesions were also examined in intra-tibially breast cancer cell injected mice and found less breast cancer cell growth and associated osteolysis in DHA-treated mice as compared to EPA-treated mice. Finally, doxorubicin-resistant MCF-7 (MCF-7dox) human breast cancer cell line was used to examine if DHA can improve sensitization of MCF-7dox cells to doxorubicin. DHA improved the inhibitory effect of doxorubicin on proliferation and invasion of MCF-7dox cells. Interestingly, drug resistance gene P-gp was also down-regulated in DHA plus doxorubicin-treated cells. In conclusion, DHA attenuates breast cancer bone metastasis and associated osteolysis more potently than EPA, possibly by inhibiting migration of breast cancer cell to the bone as well as by inhibiting osteoclastic bone resorption.
Conflict of interest statement
Figures



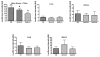

Similar articles
-
Fish oil prevents breast cancer cell metastasis to bone.Biochem Biophys Res Commun. 2010 Nov 26;402(4):602-7. doi: 10.1016/j.bbrc.2010.10.063. Epub 2010 Oct 28. Biochem Biophys Res Commun. 2010. PMID: 20971068 Free PMC article.
-
Docosahexaenoic Acid Incorporation Is Not Affected by Doxorubicin Chemotherapy in either Whole Cell or Lipid Raft Phospholipids of Breast Cancer Cells in vitro and Tumor Phospholipids in vivo.Lipids. 2020 Sep;55(5):549-565. doi: 10.1002/lipd.12252. Epub 2020 Jun 25. Lipids. 2020. PMID: 32588470
-
Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44.J Biol Chem. 2011 Apr 1;286(13):11314-27. doi: 10.1074/jbc.M110.193714. Epub 2011 Jan 3. J Biol Chem. 2011. PMID: 21199873 Free PMC article.
-
Parathyroid hormone-related protein and bone metastases.Cancer. 1997 Oct 15;80(8 Suppl):1572-80. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1572::aid-cncr7>3.3.co;2-d. Cancer. 1997. PMID: 9362424 Review.
-
Targeting the Wnt signaling pathway for breast cancer bone metastasis therapy.J Mol Med (Berl). 2022 Mar;100(3):373-384. doi: 10.1007/s00109-021-02159-y. Epub 2021 Nov 25. J Mol Med (Berl). 2022. PMID: 34821953 Review.
Cited by
-
Link between Omega 3 Fatty Acids Carried by Lipoproteins and Breast Cancer Severity.Nutrients. 2022 Jun 14;14(12):2461. doi: 10.3390/nu14122461. Nutrients. 2022. PMID: 35745191 Free PMC article.
-
Long-chain omega-3 polyunsaturated fatty acids decrease mammary tumor growth, multiorgan metastasis and enhance survival.Clin Exp Metastasis. 2018 Dec;35(8):797-818. doi: 10.1007/s10585-018-9941-7. Epub 2018 Oct 16. Clin Exp Metastasis. 2018. PMID: 30327985
-
Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line.Nutrients. 2023 Mar 29;15(7):1658. doi: 10.3390/nu15071658. Nutrients. 2023. PMID: 37049499 Free PMC article.
-
Unraveling the Omega-3 Puzzle: Navigating Challenges and Innovations for Bone Health and Healthy Aging.Mar Drugs. 2024 Sep 28;22(10):446. doi: 10.3390/md22100446. Mar Drugs. 2024. PMID: 39452854 Free PMC article. Review.
-
Omega-3 fatty acid-rich fish oil supplementation prevents rosiglitazone-induced osteopenia in aging C57BL/6 mice and in vitro studies.Sci Rep. 2021 May 14;11(1):10364. doi: 10.1038/s41598-021-89827-8. Sci Rep. 2021. PMID: 33990655 Free PMC article.
References
-
- Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm. 2009;66:1169–79. - PubMed
-
- Bagga D, Anders KH, Wang HJ, Glaspy JA. Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr Cancer. 2002;42:180–5. - PubMed
-
- Lands WE, Hamazaki T, Yamazaki K, Okuyama H, Sakai K, Goto Y, Hubbard VS. Changing dietary patterns. Am J Clin Nutr. 1990;51:991–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

