Thioredoxin 1 is inactivated due to oxidation induced by peroxiredoxin under oxidative stress and reactivated by the glutaredoxin system
- PMID: 24062305
- PMCID: PMC3820862
- DOI: 10.1074/jbc.M113.495150
Thioredoxin 1 is inactivated due to oxidation induced by peroxiredoxin under oxidative stress and reactivated by the glutaredoxin system
Abstract
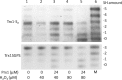
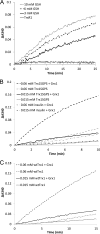
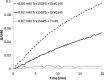
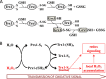
The mammalian cytosolic thioredoxin system, comprising thioredoxin (Trx), Trx reductase, and NADPH, is the major protein-disulfide reductase of the cell and has numerous functions. Besides the active site thiols, human Trx1 contains three non-active site cysteine residues at positions 62, 69, and 73. A two-disulfide form of Trx1, containing an active site disulfide between Cys-32 and Cys-35 and a non-active site disulfide between Cys-62 and Cys-69, is inactive either as a disulfide reductase or as a substrate for Trx reductase. This could possibly provide a structural switch affecting Trx1 function during oxidative stress and redox signaling. We found that two-disulfide Trx1 was generated in A549 cells under oxidative stress. In vitro data showed that two-disulfide Trx1 was generated from oxidation of Trx1 catalyzed by peroxiredoxin 1 in the presence of H2O2. The redox Western blot data indicated that the glutaredoxin system protected Trx1 in HeLa cells from oxidation caused by ebselen, a superfast oxidant for Trx1. Our results also showed that physiological concentrations of glutathione, NADPH, and glutathione reductase reduced the non-active site disulfide in vitro. This reaction was stimulated by glutaredoxin 1 via the so-called monothiol mechanism. In conclusion, reversible oxidation of the non-active site disulfide of Trx1 is suggested to play an important role in redox regulation and cell signaling via temporal inhibition of its protein-disulfide reductase activity for the transmission of oxidative signals under oxidative stress.
Keywords: Hydrogen Peroxide; Oxidative Stress; Peroxiredoxin; Redox Signaling; Thiol; Thioredoxin.
Figures


Similar articles
-
Glutathionylation of the Active Site Cysteines of Peroxiredoxin 2 and Recycling by Glutaredoxin.J Biol Chem. 2016 Feb 5;291(6):3053-62. doi: 10.1074/jbc.M115.692798. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601956 Free PMC article.
-
Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues.J Biol Chem. 2008 Aug 8;283(32):21890-8. doi: 10.1074/jbc.M801047200. Epub 2008 Jun 10. J Biol Chem. 2008. PMID: 18544525
-
Thioredoxin-related protein of 14 kDa is an efficient L-cystine reductase and S-denitrosylase.Proc Natl Acad Sci U S A. 2014 May 13;111(19):6964-9. doi: 10.1073/pnas.1317320111. Epub 2014 Apr 28. Proc Natl Acad Sci U S A. 2014. PMID: 24778250 Free PMC article.
-
Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes.Biochemistry (Mosc). 2008 Dec;73(13):1493-510. doi: 10.1134/s0006297908130099. Biochemistry (Mosc). 2008. PMID: 19216714 Review.
-
Thioredoxin and its related molecules: update 2005.Antioxid Redox Signal. 2005 May-Jun;7(5-6):823-8. doi: 10.1089/ars.2005.7.823. Antioxid Redox Signal. 2005. PMID: 15890030 Review.
Cited by
-
Diet affects the redox system in developing Atlantic cod (Gadus morhua) larvae.Redox Biol. 2015 Aug;5:308-318. doi: 10.1016/j.redox.2015.06.003. Epub 2015 Jun 12. Redox Biol. 2015. PMID: 26099546 Free PMC article.
-
TrxR1 as a potent regulator of the Nrf2-Keap1 response system.Antioxid Redox Signal. 2015 Oct 1;23(10):823-53. doi: 10.1089/ars.2015.6378. Epub 2015 Jun 24. Antioxid Redox Signal. 2015. PMID: 26058897 Free PMC article. Review.
-
Mitochondrial Thioredoxin System as a Modulator of Cyclophilin D Redox State.Sci Rep. 2016 Mar 15;6:23071. doi: 10.1038/srep23071. Sci Rep. 2016. PMID: 26975474 Free PMC article.
-
Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System.Antioxid Redox Signal. 2017 Nov 1;27(13):989-1010. doi: 10.1089/ars.2016.6925. Epub 2017 May 18. Antioxid Redox Signal. 2017. PMID: 28443683 Free PMC article. Review.
-
Monitoring thioredoxin redox with a genetically encoded red fluorescent biosensor.Nat Chem Biol. 2017 Sep;13(9):1045-1052. doi: 10.1038/nchembio.2417. Epub 2017 Jun 26. Nat Chem Biol. 2017. PMID: 28671680 Free PMC article.
References
-
- Holmgren A. (1968) Thioredoxin. 6. The amino acid sequence of the protein from Escherichia coli B. Eur. J. Biochem. 6, 475–484 - PubMed
-
- Holmgren A. (1985) Thioredoxin. Annu. Rev. Biochem. 54, 237–271 - PubMed
-
- Eklund H., Gleason F. K., Holmgren A. (1991) Structural and functional relations among thioredoxins of different species. Proteins 11, 13–28 - PubMed
-
- Holmgren A., Lu J. (2010) Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem. Biophys. Res. Commun. 396, 120–124 - PubMed
-
- Holmgren A. (2000) Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2, 811–820 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

