Redox control of the senescence regulator interleukin-1α and the secretory phenotype
- PMID: 24062309
- PMCID: PMC3820855
- DOI: 10.1074/jbc.M113.493841
Redox control of the senescence regulator interleukin-1α and the secretory phenotype
Abstract
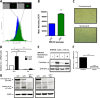
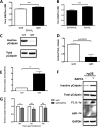
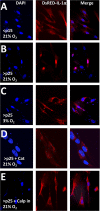
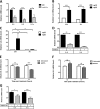
Senescent cells accumulate in aged tissue and are causally linked to age-associated tissue degeneration. These non-dividing, metabolically active cells are highly secretory and alter tissue homeostasis, creating an environment conducive to metastatic disease progression. IL-1α is a key senescence-associated (SA) proinflammatory cytokine that acts as a critical upstream regulator of the SA secretory phenotype (SASP). We established that SA shifts in steady-state H2O2 and intracellular Ca(2+) levels caused an increase in IL-1α expression and processing. The increase in intracellular Ca(2+) promoted calpain activation and increased the proteolytic cleavage of IL-1α. Antioxidants and low oxygen tension prevented SA IL-1α expression and restricted expression of SASP components IL-6 and IL-8. Ca(2+) chelation or calpain inhibition prevented SA processing of IL-1α and its ability to induce downstream cytokine expression. Conditioned medium from senescent cells treated with antioxidants or Ca(2+) chelators or cultured in low oxygen markedly reduced the invasive capacity of proximal metastatic cancer cells. In this paracrine fashion, senescent cells promoted invasion by inducing an epithelial-mesenchymal transition, actin reorganization, and cellular polarization of neighboring cancer cells. Collectively, these findings demonstrate how SA alterations in the redox state and Ca(2+) homeostasis modulate the inflammatory phenotype through the regulation of the SASP initiator IL-1α, creating a microenvironment permissive to tumor invasion.
Keywords: Antioxidants; Calcium; Calpain; Cell Invasion; Cellular Senescence; Epithelial-Mesenchymal Transition; Hydrogen Peroxide; Interleukin; Redox Regulation.
Figures


Similar articles
-
Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network.Proc Natl Acad Sci U S A. 2009 Oct 6;106(40):17031-6. doi: 10.1073/pnas.0905299106. Epub 2009 Sep 28. Proc Natl Acad Sci U S A. 2009. PMID: 19805069 Free PMC article.
-
Redox-control of the alarmin, Interleukin-1α.Redox Biol. 2013 Apr 17;1(1):218-25. doi: 10.1016/j.redox.2013.03.001. eCollection 2013. Redox Biol. 2013. PMID: 24024155 Free PMC article.
-
S100A13 promotes senescence-associated secretory phenotype and cellular senescence via modulation of non-classical secretion of IL-1α.Aging (Albany NY). 2019 Jan 23;11(2):549-572. doi: 10.18632/aging.101760. Aging (Albany NY). 2019. PMID: 30670674 Free PMC article.
-
The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions.Cancer Metastasis Rev. 2006 Sep;25(3):387-408. doi: 10.1007/s10555-006-9004-4. Cancer Metastasis Rev. 2006. PMID: 17043764 Review.
-
Evidence for calpains in cancer metastasis.J Cell Physiol. 2019 Jun;234(6):8233-8240. doi: 10.1002/jcp.27649. Epub 2018 Oct 28. J Cell Physiol. 2019. PMID: 30370545 Review.
Cited by
-
Selenoproteins and the senescence-associated epitranscriptome.Exp Biol Med (Maywood). 2022 Dec;247(23):2090-2102. doi: 10.1177/15353702221116592. Epub 2022 Aug 29. Exp Biol Med (Maywood). 2022. PMID: 36036467 Free PMC article. Review.
-
Cell Senescence: A Nonnegligible Cell State under Survival Stress in Pathology of Intervertebral Disc Degeneration.Oxid Med Cell Longev. 2020 Aug 31;2020:9503562. doi: 10.1155/2020/9503562. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32934764 Free PMC article. Review.
-
Expression of the Calcium-Binding Protein CALB1 Is Induced and Controls Intracellular Ca2+ Levels in Senescent Cells.Int J Mol Sci. 2022 Aug 19;23(16):9376. doi: 10.3390/ijms23169376. Int J Mol Sci. 2022. PMID: 36012633 Free PMC article.
-
Cold atmospheric plasma causes a calcium influx in melanoma cells triggering CAP-induced senescence.Sci Rep. 2018 Jul 3;8(1):10048. doi: 10.1038/s41598-018-28443-5. Sci Rep. 2018. PMID: 29968804 Free PMC article.
-
Anti-TNF-α treatment modulates SASP and SASP-related microRNAs in endothelial cells and in circulating angiogenic cells.Oncotarget. 2016 Mar 15;7(11):11945-58. doi: 10.18632/oncotarget.7858. Oncotarget. 2016. PMID: 26943583 Free PMC article.
References
-
- Campisi J. (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

