Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety
- PMID: 24062440
- PMCID: PMC3801051
- DOI: 10.1073/pnas.1304987110
Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety
Abstract
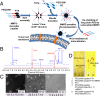
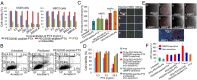
In response to the challenges of cancer chemotherapeutics, including poor physicochemical properties, low tumor targeting, insufficient tumor cell internalization/bioavailability, and side effects, we developed a unique tumor-targeted micellar drug-delivery platform. Using paclitaxel as a model therapeutic, a nanopreparation composed of a matrix metalloproteinase 2 (MMP2)-sensitive self-assembly PEG 2000-paclitaxel conjugate (as a prodrug and MMP 2-sensitive moiety), transactivating transcriptional activator peptide-PEG1000-phosphoethanolamine (PE) (a cell-penetrating enhancer), and PEG1000-PE (a nanocarrier building block) was prepared. Several major drug delivery strategies, including self-assembly, PEGylation, the enhanced permeability and retention effect, stimulus sensitivity, a cell-penetrating moiety, and the concept of prodrug, were used in design of this nanoparticle in a collaborative manner. The nanopreparation allowed superior cell internalization, cytotoxicity, tumor targeting, and antitumor efficacy in vitro and in vivo over its nonsensitive counterpart, free paclitaxel and conventional micelles. This uniquely engineered nanoparticle has potential for effective intracellular delivery of drug into cancer cells.
Keywords: multifunctional; nanomedicine; non-small cell lung cancer; polymer-drug conjugate; polymeric micelles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Well-Defined Redox-Sensitive Polyethene Glycol-Paclitaxel Prodrug Conjugate for Tumor-Specific Delivery of Paclitaxel Using Octreotide for Tumor Targeting.Mol Pharm. 2015 Aug 3;12(8):3020-31. doi: 10.1021/acs.molpharmaceut.5b00280. Epub 2015 Jun 30. Mol Pharm. 2015. PMID: 26086430
-
Enhancing cancer targeting and anticancer activity by a stimulus-sensitive multifunctional polymer-drug conjugate.J Control Release. 2015 Aug 28;212:94-102. doi: 10.1016/j.jconrel.2015.06.024. Epub 2015 Jun 22. J Control Release. 2015. PMID: 26113423
-
Building Stable MMP2-Responsive Multifunctional Polymeric Micelles by an All-in-One Polymer-Lipid Conjugate for Tumor-Targeted Intracellular Drug Delivery.ACS Appl Mater Interfaces. 2017 Sep 27;9(38):32520-32533. doi: 10.1021/acsami.7b09511. Epub 2017 Sep 13. ACS Appl Mater Interfaces. 2017. PMID: 28870072
-
Multifunctionality of lipid-core micelles for drug delivery and tumour targeting.Mol Membr Biol. 2010 Oct;27(7):232-46. doi: 10.3109/09687688.2010.516276. Epub 2010 Oct 7. Mol Membr Biol. 2010. PMID: 20929339 Review.
-
Prodrug Strategies for Paclitaxel.Int J Mol Sci. 2016 May 23;17(5):796. doi: 10.3390/ijms17050796. Int J Mol Sci. 2016. PMID: 27223283 Free PMC article. Review.
Cited by
-
Integration of Dual Targeting and Dual Therapeutic Modules Endows Self-Assembled Nanoparticles with Anti-Tumor Growth and Metastasis Functions.Int J Nanomedicine. 2021 Feb 18;16:1361-1376. doi: 10.2147/IJN.S291285. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33658777 Free PMC article.
-
Exploiting a New Approach to Destroy the Barrier of Tumor Microenvironment: Nano-Architecture Delivery Systems.Molecules. 2021 May 5;26(9):2703. doi: 10.3390/molecules26092703. Molecules. 2021. PMID: 34062992 Free PMC article. Review.
-
Intertumoral and intratumoral barriers as approaches for drug delivery and theranostics to solid tumors using stimuli-responsive materials.Mikrochim Acta. 2024 Aug 16;191(9):541. doi: 10.1007/s00604-024-06583-y. Mikrochim Acta. 2024. PMID: 39150483 Review.
-
Near-Infrared-Activated Fluorescence Resonance Energy Transfer-Based Nanocomposite to Sense MMP2-Overexpressing Oral Cancer Cells.ACS Omega. 2018 Feb 28;3(2):1627-1634. doi: 10.1021/acsomega.7b01494. Epub 2018 Feb 8. ACS Omega. 2018. PMID: 30023811 Free PMC article.
-
Enhanced and Prolonged Antitumor Effect of Salinomycin-Loaded Gelatinase-Responsive Nanoparticles via Targeted Drug Delivery and Inhibition of Cervical Cancer Stem Cells.Int J Nanomedicine. 2020 Feb 26;15:1283-1295. doi: 10.2147/IJN.S234679. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32161458 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

