Precocious cerebellum development and improved motor functions in mice lacking the astrocyte cilium-, patched 1-associated Gpr37l1 receptor
- PMID: 24062445
- PMCID: PMC3799331
- DOI: 10.1073/pnas.1314819110
Precocious cerebellum development and improved motor functions in mice lacking the astrocyte cilium-, patched 1-associated Gpr37l1 receptor
Abstract
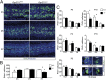
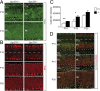
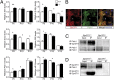
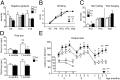
In the developing cerebellum, the proliferation and differentiation of glial and neuronal cell types depend on the modulation of the sonic hedgehog (Shh) signaling pathway. The vertebrate G-protein-coupled receptor 37-like 1 (GPR37L1) gene encodes a putative G-protein-coupled receptor that is expressed in newborn and adult cerebellar Bergmann glia astrocytes. This study shows that the ablation of the murine Gpr37l1 gene results in premature down-regulation of proliferation of granule neuron precursors and precocious maturation of Bergmann glia and Purkinje neurons. These alterations are accompanied by improved adult motor learning and coordination. Gpr37l1(-/-) mice also exhibit specific modifications of the Shh signaling cascade. Specific assays show that in Bergmann glia cells Gpr37l1 is associated with primary cilium membranes and it specifically interacts and colocalizes with the Shh primary receptor, patched 1. These findings indicate that the patched 1-associated Gpr37l1 receptor participates in the regulation of postnatal cerebellum development by modulating the Shh pathway.
Keywords: mitogenic signaling; mutant mouse model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72(5):295–339. - PubMed
-
- Gao WO, Heintz N, Hatten ME. Cerebellar granule cell neurogenesis is regulated by cell-cell interactions in vitro. Neuron. 1991;6(5):705–715. - PubMed
-
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971;141(3):283–312. - PubMed
-
- Yue Q, et al. PTEN deletion in Bergmann glia leads to premature differentiation and affects laminar organization. Development. 2005;132(14):3281–3291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

