Structural insights into proteoglycan-shaped Hedgehog signaling
- PMID: 24062467
- PMCID: PMC3799379
- DOI: 10.1073/pnas.1310097110
Structural insights into proteoglycan-shaped Hedgehog signaling
Abstract
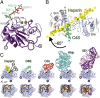
Hedgehog (Hh) morphogens play fundamental roles during embryogenesis and adulthood, in health and disease. Multiple cell surface receptors regulate the Hh signaling pathway. Among these, the glycosaminoglycan (GAG) chains of proteoglycans shape Hh gradients and signal transduction. We have determined crystal structures of Sonic Hh complexes with two GAGs, heparin and chondroitin sulfate. The interaction determinants, confirmed by site-directed mutagenesis and binding studies, reveal a previously not identified Hh site for GAG binding, common to all Hh proteins. The majority of Hh residues forming this GAG-binding site have been previously implicated in developmental diseases. Crystal packing analysis, combined with analytical ultracentrifugation of Sonic Hh-GAG complexes, suggests a potential mechanism for GAG-dependent Hh multimerization. Taken together, these results provide a direct mechanistic explanation of the observed correlation between disease and impaired Hh gradient formation. Moreover, GAG binding partially overlaps with the site of Hh interactions with an array of protein partners including Patched, hedgehog interacting protein, and the interference hedgehog protein family, suggesting a unique mechanism of Hh signaling modulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15(23):3059–3087. - PubMed
-
- Belloni E, et al. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14(3):353–356. - PubMed
-
- Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5(12):1026–1033. - PubMed
-
- Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12(6):393–406. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

