Anti-inflammatory effects of budesonide in human lung fibroblast are independent of histone deacetylase 2
- PMID: 24062615
- PMCID: PMC3780291
- DOI: 10.2147/JIR.S43736
Anti-inflammatory effects of budesonide in human lung fibroblast are independent of histone deacetylase 2
Abstract
Objective and design: Reduced expression of histone deacetylase 2 (HDAC2) in alveolar macrophages and epithelial cells may account for reduced response of chronic obstructive pulmonary disease (COPD) patients to glucocorticoids. HDAC2 expression and its role in mediating glucocorticoid effects on fibroblast functions, however, has not been fully studied. This study was designed to investigate whether HDAC2 mediates glucocorticoid effects on release of inflammatory cytokines and matrix metalloproteinases (MMPs) from human lung fibroblasts.
Methods: Human lung fibroblasts (HFL-1 cells) were stimulated with interleukin (IL)-1 β plus tumor necrosis factor (TNF)-α in the presence or absence of the glucocorticoid budesonide. Cytokines (IL-6 and IL-8) were quantified by enzyme linked immunosorbent assay (ELISA) and MMPs (MMP-1 and MMP-3) by immunoblotting in culture medium. The role of HDAC2 was investigated using a pharmacologic inhibitor as well as a small interfering ribonucleic acid (siRNA) targeting HDAC2.
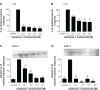
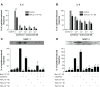
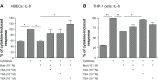
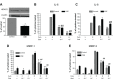
Results: We have demonstrated that budesonide concentration-dependently (10(-10)-10(-7) M) inhibited IL-6, IL-8, MMP-1, and MMP-3 release by HFL-1 cells in response to IL-1β plus TNF-α. While an HDAC inhibitor significantly blocked the inhibitory effect of budesonide on human bronchial epithelial cells (HBECs) and monocytes (THP-1 cells), it did not block the inhibitory effect of budesonide on release of cytokines and MMPs from HFL-1 cells. Similarly, an HDAC2-siRNA blocked budesonide inhibition of cytokine release in HBECs, but it did not block the inhibitory effect of budesonide on HFL-1 cytokine and MMP release. Furthermore, budesonide significantly blocked release of cytokines and MMPs to a similar degree in normal and COPD lung fibroblasts as well as in HFL-1 cells exposed or not exposed to cigarette smoke extract.
Conclusion: These findings suggest that, in contrast to airway epithelial cells and monocytes/macrophages, HDAC2 is not required for budesonide to inhibit MMP and cytokine release by lung fibroblasts and this inhibitory pathway appears to be intact in cultured fibroblasts from COPD patients. These results also suggest that budesonide has the potential to modulate fibroblast-mediated tissue remodeling following airway inflammation in COPD, which is mediated via an HDAC2 independent pathway.
Keywords: HDAC2; budesonide; fibroblasts.
Figures


Similar articles
-
Inhibitory effects of theophylline on the peroxynitrite-augmented release of matrix metalloproteinases by lung fibroblasts.Am J Physiol Lung Cell Mol Physiol. 2012 Apr 15;302(8):L764-74. doi: 10.1152/ajplung.00342.2011. Epub 2012 Jan 27. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22287608
-
Jinwei Tang modulates HDAC2 expression in a rat model of COPD.Exp Ther Med. 2018 Mar;15(3):2604-2610. doi: 10.3892/etm.2018.5707. Epub 2018 Jan 5. Exp Ther Med. 2018. PMID: 29456664 Free PMC article.
-
HDAC2 Suppresses IL17A-Mediated Airway Remodeling in Human and Experimental Modeling of COPD.Chest. 2018 Apr;153(4):863-875. doi: 10.1016/j.chest.2017.10.031. Epub 2017 Nov 4. Chest. 2018. PMID: 29113816
-
Cathelicidin LL-37 restoring glucocorticoid function in smoking and lipopolysaccharide-induced airway inflammation in rats.Chin Med J (Engl). 2019 Mar 5;132(5):569-576. doi: 10.1097/CM9.0000000000000107. Chin Med J (Engl). 2019. PMID: 30741829 Free PMC article.
-
Decreased Histone Deacetylase 2 (HDAC2) in Peripheral Blood Monocytes (PBMCs) of COPD Patients.PLoS One. 2016 Jan 25;11(1):e0147380. doi: 10.1371/journal.pone.0147380. eCollection 2016. PLoS One. 2016. PMID: 26809128 Free PMC article.
Cited by
-
Long-term treatment with budesonide/formoterol attenuates circulating CRP levels in chronic obstructive pulmonary disease patients of group D.PLoS One. 2017 Aug 23;12(8):e0183300. doi: 10.1371/journal.pone.0183300. eCollection 2017. PLoS One. 2017. PMID: 28832630 Free PMC article. Clinical Trial.
-
Pharmacotherapy of chronic obstructive pulmonary disease: Therapeutic considerations with a focus on inhaled corticosteroids.S Afr Fam Pract (2004). 2020 Dec 8;62(1):e1-e6. doi: 10.4102/safp.v62i1.5198. S Afr Fam Pract (2004). 2020. PMID: 33314947 Free PMC article.
-
Inhaled corticosteroids in COPD: Personalising the therapeutic choice.Afr J Thorac Crit Care Med. 2018 Apr 3;24(1):10.7196/AJTCCM.2018.v24i1.184. doi: 10.7196/AJTCCM.2018.v24i1.184. eCollection 2018. Afr J Thorac Crit Care Med. 2018. PMID: 34541493 Free PMC article. Review.
-
Long-term effects of acupuncture treatment on airway smooth muscle in a rat model of smoke-induced chronic obstructive pulmonary disease.Acupunct Med. 2016 Apr;34(2):107-13. doi: 10.1136/acupmed-2014-010674. Epub 2015 Sep 7. Acupunct Med. 2016. PMID: 26345700 Free PMC article.
-
Ozone Inhalation Attenuated the Effects of Budesonide on Aspergillus fumigatus-Induced Airway Inflammation and Hyperreactivity in Mice.Front Immunol. 2019 Sep 13;10:2173. doi: 10.3389/fimmu.2019.02173. eCollection 2019. Front Immunol. 2019. PMID: 31572383 Free PMC article.
References
-
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. - PubMed
-
- Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med. 2005;26(2):142–153. - PubMed
-
- Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352(19):1967–1976. - PubMed
-
- Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol. 2009;71:451–464. - PubMed
-
- Adcock IM, Ito K, Barnes PJ. Histone deacetylation: an important mechanism in inflammatory lung diseases. COPD. 2005;2(4):445–455. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

