Altered gamma oscillations during pregnancy through loss of δ subunit-containing GABA(A) receptors on parvalbumin interneurons
- PMID: 24062647
- PMCID: PMC3775147
- DOI: 10.3389/fncir.2013.00144
Altered gamma oscillations during pregnancy through loss of δ subunit-containing GABA(A) receptors on parvalbumin interneurons
Abstract
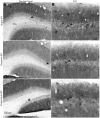
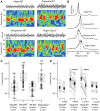
Gamma (γ) oscillations (30-120 Hz), an emergent property of neuronal networks, correlate with memory, cognition and encoding. In the hippocampal CA3 region, locally generated γ oscillations emerge through feedback between inhibitory parvalbumin-positive basket cells (PV+BCs) and the principal (pyramidal) cells. PV+BCs express δ-subunit-containing GABA(A)Rs (δ-GABA(A)Rs) and NMDA receptors (NMDA-Rs) that balance the frequency of γ oscillations. Neuroactive steroids (NS), such as the progesterone-derived (3α,5α)-3-hydroxy-pregnan-20-one (allopregnanolone; ALLO), modulate the expression of δ-GABA(A)Rs and the tonic conductance they mediate. Pregnancy produces large increases in ALLO and brain-region-specific homeostatic changes in δ-GABA(A)Rs expression. Here we show that in CA3, where most PV+ interneurons (INs) express δ-GABA(A)Rs, expression of δ-GABA(A)Rs on INs diminishes during pregnancy, but reverts to control levels within 48 h postpartum. These anatomical findings were corroborated by a pregnancy-related increase in the frequency of kainate-induced CA3 γ oscillations in vitro that could be countered by the NMDA-R antagonists D-AP5 and PPDA. Mimicking the typical hormonal conditions during pregnancy by supplementing 100 nM ALLO lowered the γ frequencies to levels found in virgin or postpartum mice. Our findings show that states of altered NS levels (e.g., pregnancy) may provoke perturbations in γ oscillatory activity through direct effects on the GABAergic system, and underscore the importance of δ-GABA(A)Rs homeostatic plasticity in maintaining constant network output despite large hormonal changes. Inaccurate coupling of NS levels to δ-GABA(A)R expression may facilitate abnormal neurological and psychiatric conditions such as epilepsy, post-partum depression, and post-partum psychosis, thus providing insights into potential new treatments.
Keywords: CA3 interneurons; GABAA receptors; delta subunit; gamma oscillations; neurosteroids; parvalbumin; pregnancy; tonic inhibition.
Figures






References
-
- Baude A., Bleasdale C., Dalezios Y., Somogyi P., Klausberger T. (2006). Immunoreactivity for the GABAA receptor alpha1 subunit, somatostatin and Connexin36 distinguishes axoaxonic, basket, and bistratified interneurons of the rat hippocampus. Cereb. Cortex 17, 2094–2107 10.1093/cercor/bhl117 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

