Factors that regulate insulin producing cells and their output in Drosophila
- PMID: 24062693
- PMCID: PMC3775311
- DOI: 10.3389/fphys.2013.00252
Factors that regulate insulin producing cells and their output in Drosophila
Abstract
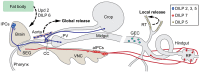
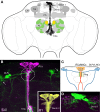
Insulin-like peptides (ILPs) and growth factors (IGFs) not only regulate development, growth, reproduction, metabolism, stress resistance, and lifespan, but also certain behaviors and cognitive functions. ILPs, IGFs, their tyrosine kinase receptors and downstream signaling components have been largely conserved over animal evolution. Eight ILPs have been identified in Drosophila (DILP1-8) and they display cell and stage-specific expression patterns. Only one insulin receptor, dInR, is known in Drosophila and most other invertebrates. Nevertheless, the different DILPs are independently regulated transcriptionally and appear to have distinct functions, although some functional redundancy has been revealed. This review summarizes what is known about regulation of production and release of DILPs in Drosophila with focus on insulin signaling in the daily life of the fly. Under what conditions are DILP-producing cells (IPCs) activated and which factors have been identified in control of IPC activity in larvae and adult flies? The brain IPCs that produce DILP2, 3 and 5 are indirectly targeted by DILP6 and a leptin-like factor from the fat body, as well as directly by a few neurotransmitters and neuropeptides. Serotonin, octopamine, GABA, short neuropeptide F (sNPF), corazonin and tachykinin-related peptide have been identified in Drosophila as regulators of IPCs. The GABAergic cells that inhibit IPCs and DILP release are in turn targeted by a leptin-like peptide (unpaired 2) from the fat body, and the IPC-stimulating corazonin/sNPF neurons may be targeted by gut-derived peptides. We also discuss physiological conditions under which IPC activity may be regulated, including nutritional states, stress and diapause induction.
Keywords: insulin receptor; insulin release; insulin signaling; metabolism; neuromodulation; neuropeptide.
Figures


Similar articles
-
Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides.Cell Mol Life Sci. 2016 Jan;73(2):271-90. doi: 10.1007/s00018-015-2063-3. Epub 2015 Oct 15. Cell Mol Life Sci. 2016. PMID: 26472340 Free PMC article. Review.
-
Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin.Cell Mol Life Sci. 2012 Dec;69(23):4051-66. doi: 10.1007/s00018-012-1097-z. Epub 2012 Jul 25. Cell Mol Life Sci. 2012. PMID: 22828865 Free PMC article.
-
Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT1A receptor.Cell Mol Life Sci. 2012 Feb;69(3):471-84. doi: 10.1007/s00018-011-0789-0. Epub 2011 Aug 5. Cell Mol Life Sci. 2012. PMID: 21818550 Free PMC article.
-
Functional implications of Drosophila insulin-like peptides in metabolism, aging, and dietary restriction.Front Physiol. 2013 Oct 16;4:288. doi: 10.3389/fphys.2013.00288. Front Physiol. 2013. PMID: 24137131 Free PMC article. Review.
-
Insulin signaling, lifespan and stress resistance are modulated by metabotropic GABA receptors on insulin producing cells in the brain of Drosophila.PLoS One. 2010 Dec 30;5(12):e15780. doi: 10.1371/journal.pone.0015780. PLoS One. 2010. PMID: 21209905 Free PMC article.
Cited by
-
A gene-specific T2A-GAL4 library for Drosophila.Elife. 2018 Mar 22;7:e35574. doi: 10.7554/eLife.35574. Elife. 2018. PMID: 29565247 Free PMC article.
-
Activating embryonic development in Drosophila.Semin Cell Dev Biol. 2018 Dec;84:100-110. doi: 10.1016/j.semcdb.2018.02.019. Epub 2018 Feb 21. Semin Cell Dev Biol. 2018. PMID: 29448071 Free PMC article. Review.
-
Endocrine and physiological regulation of neutral fat storage in Drosophila.Mol Cell Endocrinol. 2018 Feb 5;461:165-177. doi: 10.1016/j.mce.2017.09.008. Epub 2017 Sep 8. Mol Cell Endocrinol. 2018. PMID: 28893568 Free PMC article. Review.
-
The neuropeptide drosulfakinin enhances choosiness and protects males from the aging effects of social perception.Proc Natl Acad Sci U S A. 2023 Dec 19;120(51):e2308305120. doi: 10.1073/pnas.2308305120. Epub 2023 Dec 11. Proc Natl Acad Sci U S A. 2023. PMID: 38079545 Free PMC article.
-
ChIP-seq profiling of H3K4me3 and H3K27me3 in an invasive insect, Bactrocera dorsalis.Front Genet. 2023 Feb 23;14:1108104. doi: 10.3389/fgene.2023.1108104. eCollection 2023. Front Genet. 2023. PMID: 36911387 Free PMC article.
References
-
- Agrawal N., Venkiteswaran G., Sadaf S., Padmanabhan N., Banerjee S., Hasan G. (2010). Inositol 1 4, 5-trisphosphate receptor and dSTIM function in Drosophila insulin-producing neurons regulates systemic intracellular calcium homeostasis and flight. J. Neurosci. 30, 1301–1313 10.1523/JNEUROSCI.3668-09.2010 - DOI - PMC - PubMed
-
- Antonova Y., Arik A. J., Moore W., Riehle M. R., Brown M. R. (2012). Insulin-like peptides: structure, signaling, and function, in Insect Endocrinology, ed Gilbert L. I. (New York, NY: Elsevier/Academic Press; ), 63–92 10.1016/B978-0-12-384749-2.10002-0 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

