The Role of Genetic Sex in Affect Regulation and Expression of GABA-Related Genes Across Species
- PMID: 24062698
- PMCID: PMC3775314
- DOI: 10.3389/fpsyt.2013.00104
The Role of Genetic Sex in Affect Regulation and Expression of GABA-Related Genes Across Species
Abstract
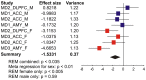
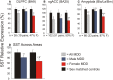
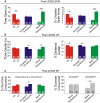
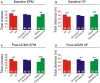
Although circulating hormones and inhibitory gamma-aminobutyric acid (GABA)-related factors are known to affect mood, considerable knowledge gaps persist for biological mechanisms underlying the female bias in mood disorders. Here, we combine human and mouse studies to investigate sexual dimorphism in the GABA system in the context of major depressive disorder (MDD) and then use a genetic model to dissect the role of sex-related factors in GABA-related gene expression and anxiety-/depressive-like behaviors in mice. First, using meta-analysis of gene array data in human postmortem brain (N = 51 MDD subjects, 50 controls), we show that the previously reported down-regulation in MDD of somatostatin (SST), a marker of a GABA neuron subtype, is significantly greater in women with MDD. Second, using gene co-expression network analysis in control human subjects (N = 214; two frontal cortex regions) and expression quantitative trait loci mapping (N = 170 subjects), we show that expression of SST and the GABA-synthesizing enzymes glutamate decarboxylase 67 (GAD67) and GAD65 are tightly co-regulated and influenced by X-chromosome genetic polymorphisms. Third, using a rodent genetic model [Four Core Genotypes (FCG) mice], in which genetic and gonadal sex are artificially dissociated (N ≥ 12/group), we show that genetic sex (i.e., X/Y-chromosome) influences both gene expression (lower Sst, Gad67, Gad65 in XY mice) and anxiety-like behaviors (higher in XY mice). This suggests that in an intact male animal, the observed behavior represents the outcomes of male genetic sex increasing and male-like testosterone decreasing anxiety-like behaviors. Gonadal sex was the only factor influencing depressive-like behavior (gonadal males < gonadal females). Collectively, these combined human and mouse studies provide mechanistic insight into sexual dimorphism in mood disorders, and specifically demonstrate an unexpected role of male-like factors (XY genetic sex) on GABA-related genes and anxiety-like behaviors.
Keywords: GABA; anxiety; depression; genetic sex; mood; somatostatin.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

