Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics
- PMID: 24062746
- PMCID: PMC3775315
- DOI: 10.3389/fimmu.2013.00280
Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics
Abstract
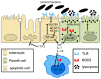
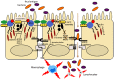
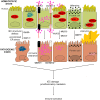
The gut mucosa is constantly challenged by a bombardment of foreign antigens and environmental microorganisms. As such, the precise regulation of the intestinal barrier allows the maintenance of mucosal immune homeostasis and prevents the onset of uncontrolled inflammation. In support of this concept, emerging evidence points to defects in components of the epithelial barrier as etiologic factors in the pathogenesis of inflammatory bowel diseases (IBDs). In fact, the integrity of the intestinal barrier relies on different elements, including robust innate immune responses, epithelial paracellular permeability, epithelial cell integrity, as well as the production of mucus. The purpose of this review is to systematically evaluate how alterations in the aforementioned epithelial components can lead to the disruption of intestinal immune homeostasis, and subsequent inflammation. In this regard, the wealth of data from mouse models of intestinal inflammation and human genetics are pivotal in understanding pathogenic pathways, for example, that are initiated from the specific loss of function of a single protein leading to the onset of intestinal disease. On the other hand, several recently proposed therapeutic approaches to treat human IBD are targeted at enhancing different elements of gut barrier function, further supporting a primary role of the epithelium in the pathogenesis of chronic intestinal inflammation and emphasizing the importance of maintaining a healthy and effective intestinal barrier.
Keywords: Crohn’s disease; animal models of intestinal inflammation; gut immune homeostasis; inflammatory bowel disease genetics; innate immunity; intestinal barrier function; intestinal epithelial cells; ulcerative colitis.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

