Organocatalyzed enantioselective desymmetrization of aziridines and epoxides
- PMID: 24062828
- PMCID: PMC3778401
- DOI: 10.3762/bjoc.9.192
Organocatalyzed enantioselective desymmetrization of aziridines and epoxides
Abstract
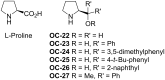
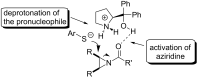
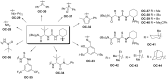
Enantioselective desymmetrization of meso-aziridines and meso-epoxides with various nucleophiles by organocatalysis has emerged as a cutting-edge approach in recent years. This review summarizes the origin and recent developments of enantioselective desymmetrization of meso-aziridines and meso-epoxides in the presence of organocatalysts.
Keywords: aziridine; desymmetrization; enantioselectivity; epoxide; organocatalysis.
Figures

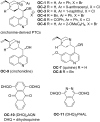

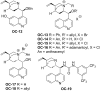
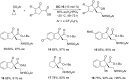
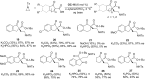
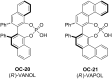
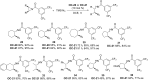
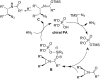

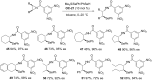


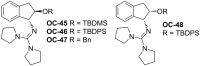
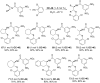
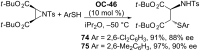
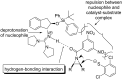
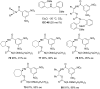


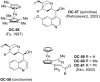
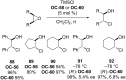
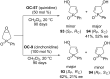
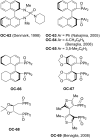
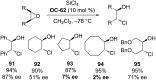

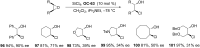
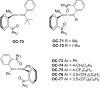

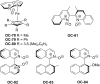





References
-
- Ojima I. Catalytic Asymmetric Synthesis. 3rd ed. Weinheim, Germany: Wiley-VCH; 2010.
-
- Bates R. Organic Synthesis Using Transition Metals. 2nd ed. West Sussex, U.K.: John Wiley & Sons; 2012.
-
- Crawley M L, Trost B M, editors. Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective. Hoboken, NJ, U.S.A.: John Wiley & Sons; 2012.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
