Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium
- PMID: 24062878
- PMCID: PMC3767075
- DOI: 10.1155/2013/474931
Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium
Abstract
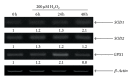
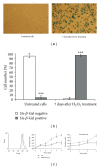
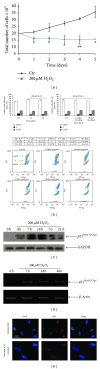
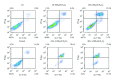
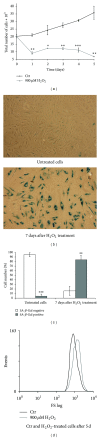
The specific responses of mesenchymal stem cells to oxidative stress may play a crucial role in regulation of tissue homeostasis as well as regeneration of organs after oxidative injury. The responses of human endometrium-derived mesenchymal stem cells (hMESCs) to oxidative stress remain still unknown. Herein, we examined the impact of H2O2 on cell viability, induction of premature senescence, and apoptosis. hMESCs were highly resistant to H2O2 compared with human diploid fibroblasts. To test a hypothesis whether hMESCs may undergo oxidative stress-induced premature senescence, cells were briefly exposed to the sublethal H2O2 doses. H2O2-treated cells were permanently arrested, lost Ki67 proliferation marker, and exhibited a senescent phenotype including cell hypertrophy and increased SA- β -Gal activity. Additionally, in stressed cells the expression levels of p21Cip1, SOD1, SOD2, and GPX1 were elevated. hMESCs survived under stress were not able to resume proliferation, indicating the irreversible loss of proliferative potential. While the low H2O2 doses promoted senescence in hMESCs, the higher H2O2 doses induced also apoptosis in a part of the cell population. Of note, senescent hMESCs exhibited high resistance to apoptosis. Thus, we have demonstrated for the first time that hMESCs may enter a state of premature senescence in response to sublethal oxidative stress.
Figures

References
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–144. - PubMed
-
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. - PubMed
-
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. Journal of Cellular Physiology. 1998;176:57–66. - PubMed
-
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nature Reviews Molecular Cell Biology. 2007;8(9):703–713. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

