Aminopeptidase N (CD13) is involved in phagocytic processes in human dendritic cells and macrophages
- PMID: 24063007
- PMCID: PMC3766993
- DOI: 10.1155/2013/562984
Aminopeptidase N (CD13) is involved in phagocytic processes in human dendritic cells and macrophages
Abstract
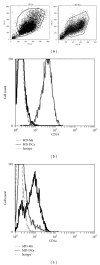
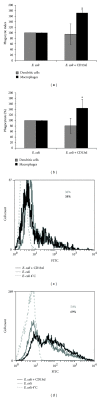
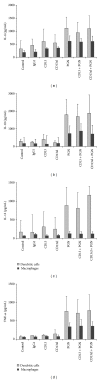
Aminopeptidase N (APN or CD13) is a membrane ectopeptidase expressed by many cell types, including myelomonocytic lineage cells: monocytes, macrophages, and dendritic cells. CD13 is known to regulate the biological activity of various peptides by proteolysis, and it has been proposed that CD13 also participates in several functions such as angiogenesis, cell adhesion, metastasis, and tumor invasion. We had previously reported that, in human monocytes and macrophages, CD13 modulates the phagocytosis mediated by receptors for the Fc portion of IgG antibodies (FcγRs). In this work, we analyzed the possible interaction of CD13 with other phagocytic receptors. We found out that the cross-linking of CD13 positively modulates the phagocytosis mediated by receptors of the innate immune system, since a significant increase in the phagocytosis of zymosan particles or heat-killed E. coli was observed when CD13 was cross-linked using anti-CD13 antibodies, in both macrophages and dendritic cells. Also, we observed that, during the phagocytosis of zymosan, CD13 redistributes and is internalized into the phagosome. These findings suggest that, besides its known functions, CD13 participates in phagocytic processes in dendritic cells and macrophages.
Figures



Similar articles
-
CD13 mediates phagocytosis in human monocytic cells.J Leukoc Biol. 2015 Jul;98(1):85-98. doi: 10.1189/jlb.2A0914-458R. Epub 2015 May 1. J Leukoc Biol. 2015. PMID: 25934926 Free PMC article.
-
Aminopeptidase N (CD13) functionally interacts with FcgammaRs in human monocytes.J Leukoc Biol. 2005 Jun;77(6):1008-17. doi: 10.1189/jlb.1204714. Epub 2005 Mar 9. J Leukoc Biol. 2005. PMID: 15758076 Free PMC article.
-
IL-10 and TGF-beta differ in their regulation of aminopeptidase N/CD13 expression in monocytes.Int J Mol Med. 2004 Jun;13(6):877-82. doi: 10.3892/ijmm.13.6.877. Int J Mol Med. 2004. PMID: 15138629
-
CD13/Aminopeptidase N Is a Potential Therapeutic Target for Inflammatory Disorders.J Immunol. 2020 Jan 1;204(1):3-11. doi: 10.4049/jimmunol.1900868. J Immunol. 2020. PMID: 31848300 Free PMC article. Review.
-
Development of monocytes, macrophages, and dendritic cells.Science. 2010 Feb 5;327(5966):656-61. doi: 10.1126/science.1178331. Science. 2010. PMID: 20133564 Free PMC article. Review.
Cited by
-
Leukocyte Membrane Enzymes Play the Cell Adhesion Game.Front Immunol. 2021 Nov 23;12:742292. doi: 10.3389/fimmu.2021.742292. eCollection 2021. Front Immunol. 2021. PMID: 34887854 Free PMC article. Review.
-
Localization, Shedding, Regulation and Function of Aminopeptidase N/CD13 on Fibroblast like Synoviocytes.PLoS One. 2016 Sep 22;11(9):e0162008. doi: 10.1371/journal.pone.0162008. eCollection 2016. PLoS One. 2016. PMID: 27658265 Free PMC article.
-
Antibody-Mediated Targeting of Antigens to Intestinal Aminopeptidase N Elicits Gut IgA Responses in Pigs.Front Immunol. 2021 Oct 14;12:753371. doi: 10.3389/fimmu.2021.753371. eCollection 2021. Front Immunol. 2021. PMID: 34721427 Free PMC article.
-
Analysis of the immune response of human dendritic cells to Mycobacterium tuberculosis by quantitative proteomics.Proteome Sci. 2016 Mar 8;14:5. doi: 10.1186/s12953-016-0095-8. eCollection 2016. Proteome Sci. 2016. PMID: 26957948 Free PMC article.
-
Proteomic analysis of urine exosomes reveals renal tubule response to leptospiral colonization in experimentally infected rats.PLoS Negl Trop Dis. 2015 Mar 20;9(3):e0003640. doi: 10.1371/journal.pntd.0003640. eCollection 2015 Mar. PLoS Negl Trop Dis. 2015. PMID: 25793258 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

