Functional studies of Drosophila zinc transporters reveal the mechanism for dietary zinc absorption and regulation
- PMID: 24063361
- PMCID: PMC4015762
- DOI: 10.1186/1741-7007-11-101
Functional studies of Drosophila zinc transporters reveal the mechanism for dietary zinc absorption and regulation
Abstract
Background: Zinc is key to the function of many proteins, but the process of dietary zinc absorption is not well clarified. Current knowledge about dietary zinc absorption is fragmented, and mostly derives from incomplete mammalian studies. To gain a comprehensive picture of this process, we systematically characterized all zinc transporters (that is, the Zip and ZnT family members) for their possible roles in dietary zinc absorption in a genetically amenable model organism, Drosophila melanogaster.
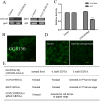
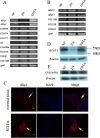
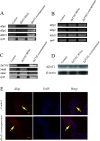
Results: A set of plasma membrane-resident zinc transporters was identified to be responsible for absorbing zinc from the lumen into the enterocyte and the subsequent exit of zinc to the circulation. dZip1 and dZip2, two functionally overlapping zinc importers, are responsible for absorbing zinc from the lumen into the enterocyte. Exit of zinc to the circulation is mediated through another two functionally overlapping zinc exporters, dZnT1, and its homolog CG5130 (dZnT77C). Somewhat surprisingly, it appears that the array of intracellular ZnT proteins, including the Golgi-resident dZnT7, is not directly involved in dietary zinc absorption. By modulating zinc status in different parts of the body, we found that regulation of dietary zinc absorption, in contrast to that of iron, is unresponsive to bodily needs or zinc status outside the gut. The zinc transporters that are involved in dietary zinc absorption, including the importers dZip1 and dZip2, and the exporter dZnT1, are respectively regulated at the RNA and protein levels by zinc in the enterocyte.
Conclusions: Our study using the model organism Drosophila thus starts to reveal a comprehensive sketch of dietary zinc absorption and its regulatory control, a process that is still incompletely understood in mammalian organisms. The knowledge gained will act as a reference for future mammalian studies, and also enable an appreciation of this important process from an evolutionary perspective.
Figures

Similar articles
-
Dietary zinc absorption is mediated by ZnT1 in Drosophila melanogaster.FASEB J. 2009 Aug;23(8):2650-61. doi: 10.1096/fj.08-126649. Epub 2009 Mar 26. FASEB J. 2009. PMID: 19325039
-
Drosophila ZnT1 is essential in the intestine for dietary zinc absorption.Biochem Biophys Res Commun. 2020 Dec 17;533(4):1004-1011. doi: 10.1016/j.bbrc.2020.09.077. Epub 2020 Oct 1. Biochem Biophys Res Commun. 2020. PMID: 33012507
-
Compartmentalized zinc deficiency and toxicities caused by ZnT and Zip gene over expression result in specific phenotypes in Drosophila.Int J Biochem Cell Biol. 2015 Mar;60:23-33. doi: 10.1016/j.biocel.2014.12.017. Epub 2015 Jan 3. Int J Biochem Cell Biol. 2015. PMID: 25562517
-
A fly's eye view of zinc homeostasis: Novel insights into the genetic control of zinc metabolism from Drosophila.Arch Biochem Biophys. 2016 Dec 1;611:142-149. doi: 10.1016/j.abb.2016.07.015. Epub 2016 Jul 22. Arch Biochem Biophys. 2016. PMID: 27453039 Review.
-
Gastrointestinal factors influencing zinc absorption and homeostasis.Int J Vitam Nutr Res. 2010 Oct;80(4-5):243-8. doi: 10.1024/0300-9831/a000030. Int J Vitam Nutr Res. 2010. PMID: 21462106 Free PMC article. Review.
Cited by
-
Biogenesis of zinc storage granules in Drosophila melanogaster.J Exp Biol. 2018 Mar 19;221(Pt 6):jeb168419. doi: 10.1242/jeb.168419. J Exp Biol. 2018. PMID: 29367274 Free PMC article.
-
ZnT7 RNAi favors RafGOFscrib-/--induced tumor growth and invasion in Drosophila through JNK signaling pathway.Oncogene. 2021 Mar;40(12):2217-2229. doi: 10.1038/s41388-021-01703-x. Epub 2021 Mar 1. Oncogene. 2021. PMID: 33649534
-
A role for the Drosophila zinc transporter Zip88E in protecting against dietary zinc toxicity.PLoS One. 2017 Jul 13;12(7):e0181237. doi: 10.1371/journal.pone.0181237. eCollection 2017. PLoS One. 2017. PMID: 28704512 Free PMC article.
-
Restoration of the GTPase activity and cellular interactions of Gαo mutants by Zn2+ in GNAO1 encephalopathy models.Sci Adv. 2022 Oct 7;8(40):eabn9350. doi: 10.1126/sciadv.abn9350. Epub 2022 Oct 7. Sci Adv. 2022. PMID: 36206333 Free PMC article.
-
Elemental mapping of the entire intact Drosophila gastrointestinal tract.J Biol Inorg Chem. 2015 Sep;20(6):979-87. doi: 10.1007/s00775-015-1281-3. Epub 2015 Jul 8. J Biol Inorg Chem. 2015. PMID: 26153547
References
-
- MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;11:1500S–1508S. - PubMed
-
- da Silva JJRF, Williams RJP. The Biological Chemistry of the Elements. Oxford: Oxford University Press; 2001. Zinc: Lewis acid catalysis and regulation; pp. 315–335.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

