Divergent mechanisms for enzymatic excision of 5-formylcytosine and 5-carboxylcytosine from DNA
- PMID: 24063363
- PMCID: PMC3930231
- DOI: 10.1021/ja406444x
Divergent mechanisms for enzymatic excision of 5-formylcytosine and 5-carboxylcytosine from DNA
Abstract
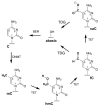
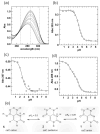
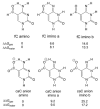
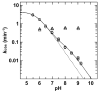
5-Methylcytosine (mC) is an epigenetic mark that impacts transcription, development, and genome stability, and aberrant DNA methylation contributes to aging and cancer. Active DNA demethylation involves stepwise oxidation of mC to 5-hydroxymethylcytosine, 5-formylcytosine (fC), and potentially 5-carboxylcytosine (caC), excision of fC or caC by thymine DNA glycosylase (TDG), and restoration of cytosine via follow-on base excision repair. Here, we investigate the mechanism for TDG excision of fC and caC. We find that 5-carboxyl-2'-deoxycytidine ionizes with pK(a) values of 4.28 (N3) and 2.45 (carboxyl), confirming that caC exists as a monoanion at physiological pH. Calculations do not support the proposal that G·fC and G·caC base pairs adopt a wobble structure that is recognized by TDG. Previous studies show that N-glycosidic bond hydrolysis follows a stepwise (S(N)1) mechanism, and that TDG activity increases with pyrimidine N1 acidity, that is, leaving group quality of the target base. Calculations here show that fC and the neutral tautomers of caC are acidic relative to other TDG substrates, but the caC monoanion exhibits poor acidity and likely resists TDG excision. While fC activity is independent of pH, caC excision is acid-catalyzed, and the pH profile indicates that caC ionizes in the enzyme-substrate complex with an apparent pKa of 5.8, likely at N3. Mutational analysis reveals that Asn191 is essential for excision of caC but dispensable for fC activity, indicating that N191 may stabilize N3-protonated forms of caC to facilitate acid catalysis and suggesting that N191A-TDG could potentially be useful for studying DNA demethylation in cells.
Figures





References
-
- Jones PA, Baylin SB. Nat Rev Genet. 2002;3:415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

