Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin
- PMID: 24063408
- PMCID: PMC3849358
- DOI: 10.1186/1475-2840-12-136
Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin
Abstract
Background: To study the pathogenesis of diabetic cardiomyopathy, reliable animal models of type 2 diabetes are required. Physiologically relevant rodent models are needed, which not only replicate the human pathology but also mimic the disease process. Here we characterised cardiac metabolic abnormalities, and investigated the optimal experimental approach for inducing disease, in a new model of type 2 diabetes.
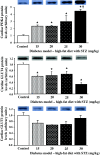
Methods and results: Male Wistar rats were fed a high-fat diet for three weeks, with a single intraperitoneal injection of low dose streptozotocin (STZ) after fourteen days at 15, 20, 25 or 30 mg/kg body weight. Compared with chow-fed or high-fat diet fed control rats, a high-fat diet in combination with doses of 15-25 mg/kg STZ did not change insulin concentrations and rats maintained body weight. In contrast, 30 mg/kg STZ induced hypoinsulinaemia, hyperketonaemia and weight loss. There was a dose-dependent increase in blood glucose and plasma lipids with increasing concentrations of STZ. Cardiac and hepatic triglycerides were increased by all doses of STZ, in contrast, cardiac glycogen concentrations increased in a dose-dependent manner with increasing STZ concentrations. Cardiac glucose transporter 4 protein levels were decreased, whereas fatty acid metabolism-regulated proteins, including uncoupling protein 3 and pyruvate dehydrogenase (PDH) kinase 4, were increased with increasing doses of STZ. Cardiac PDH activity displayed a dose-dependent relationship between enzyme activity and STZ concentration. Cardiac insulin-stimulated glycolytic rates were decreased by 17% in 15 mg/kg STZ high-fat fed diabetic rats compared with control rats, with no effect on cardiac contractile function.
Conclusions: High-fat feeding in combination with a low dose of STZ induced cardiac metabolic changes that mirror the decrease in glucose metabolism and increase in fat metabolism in diabetic patients. While low doses of 15-25 mg/kg STZ induced a type 2 diabetic phenotype, higher doses more closely recapitulated type 1 diabetes, demonstrating that the severity of diabetes can be modified according to the requirements of the study.
Figures





References
-
- Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–S21. - PubMed
-
- McGill JB, Peterson LR, Herrero P, Saeed IM, Recklein C, Coggan AR, Demoss AJ, Schechtman KB, Dence CS, Gropler RJ. Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. J Nucl Cardiol. 2011;18(3):421–429. doi: 10.1007/s12350-011-9362-3. quiz 432–423. - DOI - PMC - PubMed
-
- Rijzewijk LJ, van der Meer RW, Lamb HJ, De Jong HW, Lubberink M, Romijn JA, Bax JJ, De Roos A, Twisk JW, Heine RJ. et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54(16):1524–1532. doi: 10.1016/j.jacc.2009.04.074. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

