Bioorthogonal layer-by-layer encapsulation of pancreatic islets via hyperbranched polymers
- PMID: 24063764
- PMCID: PMC3856945
- DOI: 10.1021/am401981g
Bioorthogonal layer-by-layer encapsulation of pancreatic islets via hyperbranched polymers
Abstract
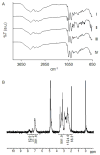
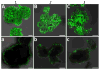
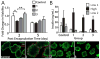
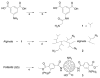
Encapsulation of viable tissues via layer-by-layer polymer assembly provides a versatile platform for cell surface engineering, with nanoscale control over the capsule properties. Herein, we report the development of a hyperbranched polymer-based, ultrathin capsule architecture expressing bioorthogonal functionality and tailored physiochemical properties. Random carbodiimide-based condensation of 3,5-dicarboxyphenyl glycineamide on alginate yielded a highly branched polysaccharide with multiple, spatially restricted, and readily functionalizable terminal carboxylate moieties. Poly(ethylene glycol) (PEG) was utilized to link azido end groups to the structured alginate. Together with a phosphine-functionalized poly(amidoamine) dendrimer, nanoscale layer-by-layer coatings, covalently stabilized via Staudinger ligation, were assembled onto solid surfaces and pancreatic islets. The effects of electrostatic and/or bioorthogonal covalent interlayer interactions on the resulting coating efficiency and stability, as well as pancreatic islet viability and function, were studied. These hyperbranched polymers provide a flexible platform for the formation of covalently stabilized, ultrathin coatings on viable cells and tissues. In addition, the hyperbranched nature of the polymers presents a highly functionalized surface capable of bioorthogonal conjugation of additional bioactive or labeling motifs.
Figures







Similar articles
-
Promoting Dendrimer Self-Assembly Enhances Covalent Layer-by-Layer Encapsulation of Pancreatic Islets.ACS Biomater Sci Eng. 2020 May 11;6(5):2641-2651. doi: 10.1021/acsbiomaterials.9b01033. Epub 2019 Nov 7. ACS Biomater Sci Eng. 2020. PMID: 32587885 Free PMC article.
-
Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via Staudinger ligation.Acta Biomater. 2011 Feb;7(2):614-24. doi: 10.1016/j.actbio.2010.07.016. Epub 2010 Jul 21. Acta Biomater. 2011. PMID: 20654745 Free PMC article.
-
Antifibrotic effect of rapamycin containing polyethylene glycol-coated alginate microcapsule in islet xenotransplantation.J Tissue Eng Regen Med. 2017 Apr;11(4):1274-1284. doi: 10.1002/term.2029. Epub 2015 Jun 5. J Tissue Eng Regen Med. 2017. PMID: 26043934
-
Patterning of hyperbranched polymer films.Chemphyschem. 2001 Nov 19;2(11):644-54. doi: 10.1002/1439-7641(20011119)2:11<644::AID-CPHC644>3.0.CO;2-#. Chemphyschem. 2001. PMID: 23686898 Review.
-
Polymers in cell encapsulation from an enveloped cell perspective.Adv Drug Deliv Rev. 2014 Apr;67-68:15-34. doi: 10.1016/j.addr.2013.11.005. Epub 2013 Nov 22. Adv Drug Deliv Rev. 2014. PMID: 24270009 Review.
Cited by
-
Visible light-initiated interfacial thiol-norbornene photopolymerization for forming islet surface conformal coating.J Mater Chem B. 2015;3(2):170-175. doi: 10.1039/C4TB01593B. J Mater Chem B. 2015. PMID: 26509035 Free PMC article.
-
Designing biomaterials for the modulation of allogeneic and autoimmune responses to cellular implants in Type 1 Diabetes.Acta Biomater. 2021 Oct 1;133:87-101. doi: 10.1016/j.actbio.2021.05.039. Epub 2021 Jun 5. Acta Biomater. 2021. PMID: 34102338 Free PMC article. Review.
-
An anionic, endosome-escaping polymer to potentiate intracellular delivery of cationic peptides, biomacromolecules, and nanoparticles.Nat Commun. 2019 Nov 1;10(1):5012. doi: 10.1038/s41467-019-12906-y. Nat Commun. 2019. PMID: 31676764 Free PMC article.
-
Pig-to-Primate Islet Xenotransplantation: Past, Present, and Future.Cell Transplant. 2017 Jun 9;26(6):925-947. doi: 10.3727/096368917X694859. Epub 2017 Feb 3. Cell Transplant. 2017. PMID: 28155815 Free PMC article. Review.
-
Promoting Dendrimer Self-Assembly Enhances Covalent Layer-by-Layer Encapsulation of Pancreatic Islets.ACS Biomater Sci Eng. 2020 May 11;6(5):2641-2651. doi: 10.1021/acsbiomaterials.9b01033. Epub 2019 Nov 7. ACS Biomater Sci Eng. 2020. PMID: 32587885 Free PMC article.
References
-
- Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior PA, Maffi P, Posselt A, Stock PG, Kaufman DB, Luo X, Kandeel F, Cagliero E, Turgeon NA, Witkowski P, Naji A, O’Connell PJ, Greenbaum C, Kudva YC, Brayman KL, Aull MJ, Larsen C, Kay TW, Fernandez LA, Vantyghem MC, Bellin M, Shapiro AM. Diabetes Care. 2012;35:1436–45. - PMC - PubMed
-
- Teramura Y, Iwata H. Adv Drug Del Rev. 2010;62:827–40. - PubMed
-
- Scharp DW, Marchetti P. Adv Drug Del Rev. 2013 in press. - PubMed
-
- Lim F, Sun A. Science. 1980;210:908–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

