Anti-wrinkle effect of bone morphogenetic protein receptor 1a-extracellular domain (BMPR1a-ECD)
- PMID: 24064062
- PMCID: PMC4133875
- DOI: 10.5483/bmbrep.2013.46.9.238
Anti-wrinkle effect of bone morphogenetic protein receptor 1a-extracellular domain (BMPR1a-ECD)
Abstract
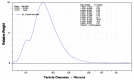
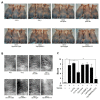
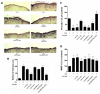
Bone morphogenetic proteins (BMPs) have diverse and important roles in the proliferation and differentiation of adult stem cells in our tissues. Especially, BMPs are well known to be the main inducers of bone formation, by facilitating both proliferation and differentiation of bone stem cells. Interestingly, in skin stem cells, BMPs repress their proliferation but are indispensable for the proper differentiation into several lineages of skin cells. Here, we tested whether BMP antagonists have an effect on the prevention of wrinkle formation. For this study we used an in vivo wrinkle-induced mouse model. As a positive control, retinoic acid, one of the top anti-wrinkle effectors, showed a 44% improvement compared to the non-treated control. Surprisingly, bone morphogenetic protein receptor 1a extracellular domain (BMPR1a-ECD) exhibited an anti-wrinkle effect which was 6-fold greater than that of retinoic acid. Our results indicate that BMP antagonists will be good targets for skin or hair diseases.
[BMB Reports 2013; 46(9): 465-470].
Figures

Similar articles
-
The bone morphogenetic protein receptor-1A pathway is required for lactogenic differentiation of mammary epithelial cells in vitro.In Vitro Cell Dev Biol Anim. 2012 Jun;48(6):377-84. doi: 10.1007/s11626-012-9522-z. Epub 2012 Jun 23. In Vitro Cell Dev Biol Anim. 2012. PMID: 22729646 Free PMC article.
-
Bone morphogenetic protein 2 stimulates noncanonical SMAD2/3 signaling via the BMP type 1A receptor in gonadotrope-like cells: implications for FSH synthesis.Endocrinology. 2014 May;155(5):1970-81. doi: 10.1210/en.2013-1741. Epub 2014 Mar 6. Endocrinology. 2014. PMID: 24601881
-
Structure of the Alk1 extracellular domain and characterization of its bone morphogenetic protein (BMP) binding properties.Biochemistry. 2012 Aug 14;51(32):6328-41. doi: 10.1021/bi300942x. Epub 2012 Aug 2. Biochemistry. 2012. PMID: 22799562 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Bone Morphogenetic Proteins: Promising Molecules for Bone Healing, Bioengineering, and Regenerative Medicine.Vitam Horm. 2015;99:293-322. doi: 10.1016/bs.vh.2015.06.002. Epub 2015 Jul 15. Vitam Horm. 2015. PMID: 26279381 Review.
Cited by
-
Acceleration of heat shock-induced collagen breakdown in human dermal fibroblasts with knockdown of NF-E2-related factor 2.BMB Rep. 2015 Aug;48(8):467-72. doi: 10.5483/bmbrep.2015.48.8.215. BMB Rep. 2015. PMID: 25441422 Free PMC article.
-
Bone Morphogenetic Protein-Based Therapeutic Approaches.Cold Spring Harb Perspect Biol. 2018 Apr 2;10(4):a022327. doi: 10.1101/cshperspect.a022327. Cold Spring Harb Perspect Biol. 2018. PMID: 28389444 Free PMC article. Review.
-
A Survey of Strategies to Modulate the Bone Morphogenetic Protein Signaling Pathway: Current and Future Perspectives.Stem Cells Int. 2016;2016:7290686. doi: 10.1155/2016/7290686. Epub 2016 Jun 28. Stem Cells Int. 2016. PMID: 27433166 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

