The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages
- PMID: 24064148
- PMCID: PMC3797185
- DOI: 10.1016/j.biomaterials.2013.08.015
The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages
Abstract
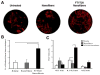
Endogenous signals originating at the site of injury are involved in the paracrine recruitment, proliferation, and differentiation of circulating progenitor and diverse inflammatory cell types. Here, we investigate a strategy to exploit endogenous cell recruitment mechanisms to regenerate injured bone by local targeting and activation of sphingosine-1-phosphate (S1P) receptors. A mandibular defect model was selected for evaluating regeneration of bone following trauma or congenital disease. The particular challenges of mandibular reconstruction are inherent in the complex anatomy and function of the bone given that the area is highly vascularized and in close proximity to muscle. Nanofibers composed of poly(DL-lactide-co-glycolide) (PLAGA) and polycaprolactone (PCL) were used to delivery FTY720, a targeted agonist of S1P receptors 1 and 3. In vitro culture of bone progenitor cells on drug-loaded constructs significantly enhanced SDF1α mediated chemotaxis of bone marrow mononuclear cells. In vivo results show that local delivery of FTY720 from composite nanofibers enhanced blood vessel ingrowth and increased recruitment of M2 alternatively activated macrophages, leading to significant osseous tissue ingrowth into critical sized defects after 12 weeks of treatment. These results demonstrate that local activation of S1P receptors is a regenerative cue resulting in recruitment of wound healing or anti-inflammatory macrophages and bone healing. Use of such small molecule therapy can provide an alternative to biological factors for the clinical treatment of critical size craniofacial defects.
Keywords: Bone healing; Craniofacial reconstruction; FTY720; Neovascularization; S1P.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


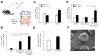
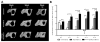


References
-
- Maurer P, Eckert AW, Kriwalsky MS, Schubert J. Scope and limitations of methods of mandibular reconstruction: a long-term follow-up. Brit J Oral Maxill. 2010;48(2):100–4. - PubMed
-
- Mariani PB, Kowalski LP, Magrin J. Reconstruction of large defects postmandibulectomy for oral cancer using plate and myocutaneous flaps: a long-term follow-up. Int J Oral Maxillofac Surg. 2006;35(5):427–32. - PubMed
-
- Schliephake H, Weich HA, Dullin C, Gruber R, Frahse S. Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid--an experimental study in rats. Biomaterials. 2008;29(1):103–10. - PubMed
-
- Herford AS, Lu M, Buxton AN, Kim J, Henkin J, Boyne PJ, Caruso JM, Rungcharassaeng K, Hong J. Recombinant human bone morphogenetic protein 2 combined with an osteoconductive bulking agent for mandibular continuity defects in nonhuman primates. J Oral Maxillofac Surg. 2012;70(3):703–16. - PubMed
-
- Zétola A, Ferreira FM, Larson R, Shibli JA. Recombinant human bone morphogenetic protein-2 (rhBMP-2) in the treatment of mandibular sequelae after tumor resection. Oral Maxillofac Surg. 2011;15(3):169–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

