Characterization of a membrane-active peptide from the Bordetella pertussis CyaA toxin
- PMID: 24064217
- PMCID: PMC3820891
- DOI: 10.1074/jbc.M113.508838
Characterization of a membrane-active peptide from the Bordetella pertussis CyaA toxin
Abstract
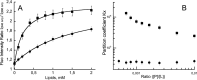
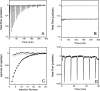
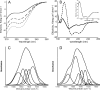
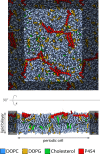
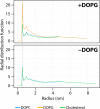
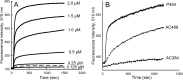
Bordetella pertussis, the pathogenic bacteria responsible for whooping cough, secretes several virulence factors, among which is the adenylate cyclase toxin (CyaA) that plays a crucial role in the early stages of human respiratory tract colonization. CyaA invades target cells by translocating its catalytic domain directly across the plasma membrane and overproduces cAMP, leading to cell death. The molecular process leading to the translocation of the catalytic domain remains largely unknown. We have previously shown that the catalytic domain per se, AC384, encompassing residues 1-384 of CyaA, did not interact with lipid bilayer, whereas a longer polypeptide, AC489, spanning residues 1-489, binds to membranes and permeabilizes vesicles. Moreover, deletion of residues 375-485 within CyaA abrogated the translocation of the catalytic domain into target cells. Here, we further identified within this region a peptidic segment that exhibits membrane interaction properties. A synthetic peptide, P454, corresponding to this sequence (residues 454-485 of CyaA) was characterized by various biophysical approaches. We found that P454 (i) binds to membranes containing anionic lipids, (ii) adopts an α-helical structure oriented in plane with respect to the lipid bilayer, and (iii) permeabilizes vesicles. We propose that the region encompassing the helix 454-485 of CyaA may insert into target cell membrane and induce a local destabilization of the lipid bilayer, thus favoring the translocation of the catalytic domain across the plasma membrane.
Keywords: Adenylate Cyclase (Adenylyl Cyclase); Bacterial Toxins; Circular Dichroism (CD); Infrared Spectroscopy; Membrane; Molecular Dynamics; Peptide Conformation; Phospholipid Vesicle.
Figures

References
-
- Ladant D., Brezin C., Alonso J. M., Crenon I., Guiso N. (1986) Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J. Biol. Chem. 261, 16264–16269 - PubMed
-
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. (1988) The calmodulin-sensitive adenylate cyclase of Bordetella pertussis. Cloning and expression in Escherichia coli. Mol. Microbiol. 2, 19–30 - PubMed
-
- Ladant D., Ullmann A. (1999) Bordatella pertussis adenylate cyclase. A toxin with multiple talents. Trends Microbiol. 7, 172–176 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

