Regulation of LH receptor mRNA binding protein by miR-122 in rat ovaries
- PMID: 24064360
- PMCID: PMC3836072
- DOI: 10.1210/en.2013-1619
Regulation of LH receptor mRNA binding protein by miR-122 in rat ovaries
Abstract
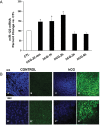
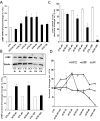
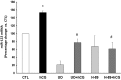
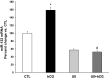
LH receptor (LHR) expression in the ovary is regulated by the RNA binding protein, (LHR mRNA binding protein [LRBP]), which has been identified as being mevalonate kinase. This study examined the role of microRNA miR-122 in LRBP-mediated LHR mRNA expression. Real-time PCR analysis of ovaries from pregnant mare serum gonadotropin/human chorionic gonadotropin (hCG)-primed female rats treated with hCG to down-regulate LHR expression showed that an increase in miR-122 expression preceded LHR mRNA down-regulation. The expression of miR-122 and its regulation was confirmed using fluorescent in situ hybridization of the frozen ovary sections using 5'-fluorescein isothiocyanate-labeled miR-122 locked nucleic acid probe. The increased expression of miR-122 preceded increased expression of LRBP mRNA and protein, and these increases were followed by LHR mRNA down-regulation. Inhibition of protein kinase A (PKA) and ERK1/2 signaling pathways by H89 and UO126, respectively, attenuated the hCG-mediated up-regulation of miR-122 levels. This was also confirmed in vitro using human granulosa cells. These results suggest the possibility that hCG-mediated miR-122 expression is mediated by the activation of cAMP/PKA/ERK signaling pathways. Inhibition of miR-122 by injection of the locked nucleic acid-conjugated antagomir of miR-122 abrogated the hCG-mediated increases in LRBP protein expression. Because it has been previously shown that miR-122 regulates sterol regulatory element-binding proteins (SREBPs) and SREBPs, in turn, regulate LRBP expression, the role of SREBPs in miR-122-mediated increase in LRBP expression was then examined. The levels of active forms of both SREBP-1a and SREBP-2 were increased in response to hCG treatment, and the stimulatory effect was sustained up to 4 hours. Taken together, our results suggest that hCG-induced down-regulation of LHR mRNA expression is mediated by activation of cAMP/PKA/ERK pathways to increase miR-122 expression, which then increases LRBP expression through the activation of SREBPs.
Figures


Comment in
-
MicroRNA-122: a new player in the negative regulation of LH receptor expression by the LH receptor mRNA binding protein (LRBP).Endocrinology. 2013 Dec;154(12):4439-42. doi: 10.1210/en.2013-1897. Endocrinology. 2013. PMID: 24273230 Free PMC article. No abstract available.
Similar articles
-
Regulation of Luteinizing Hormone Receptor mRNA Expression in the Ovary: The Role of miR-122.Vitam Horm. 2018;107:67-87. doi: 10.1016/bs.vh.2018.01.010. Epub 2018 Feb 19. Vitam Horm. 2018. PMID: 29544643 Free PMC article. Review.
-
miR-122 Regulates LH Receptor Expression by Activating Sterol Response Element Binding Protein in Rat Ovaries.Endocrinology. 2015 Sep;156(9):3370-80. doi: 10.1210/en.2015-1121. Epub 2015 Jun 30. Endocrinology. 2015. PMID: 26125464 Free PMC article.
-
Luteinizing hormone receptor mRNA down-regulation is mediated through ERK-dependent induction of RNA binding protein.Mol Endocrinol. 2011 Feb;25(2):282-90. doi: 10.1210/me.2010-0366. Epub 2010 Dec 8. Mol Endocrinol. 2011. PMID: 21147848 Free PMC article.
-
Hypusination of eukaryotic initiation factor 5A via cAMP-PKA-ERK1/2 pathway is required for ligand-induced downregulation of LH receptor mRNA expression in the ovary.Mol Cell Endocrinol. 2015 Sep 15;413:90-5. doi: 10.1016/j.mce.2015.06.014. Epub 2015 Jun 23. Mol Cell Endocrinol. 2015. PMID: 26116232 Free PMC article.
-
Regulation of luteinizing hormone receptor expression by an RNA binding protein: role of ERK signaling.Indian J Med Res. 2014 Nov;140 Suppl(Suppl 1):S112-9. Indian J Med Res. 2014. PMID: 25673531 Free PMC article. Review.
Cited by
-
IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells.J Clin Endocrinol Metab. 2014 Aug;99(8):2995-3004. doi: 10.1210/jc.2014-1139. Epub 2014 May 21. J Clin Endocrinol Metab. 2014. PMID: 24848710 Free PMC article.
-
Updates on molecular and environmental determinants of luteal progesterone production.Mol Cell Endocrinol. 2020 Sep 15;515:110930. doi: 10.1016/j.mce.2020.110930. Epub 2020 Jun 28. Mol Cell Endocrinol. 2020. PMID: 32610113 Free PMC article. Review.
-
The Role of microRNAs in Ovarian Granulosa Cells in Health and Disease.Front Endocrinol (Lausanne). 2019 Mar 21;10:174. doi: 10.3389/fendo.2019.00174. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30949134 Free PMC article. Review.
-
Regulation of Luteinizing Hormone Receptor mRNA Expression in the Ovary: The Role of miR-122.Vitam Horm. 2018;107:67-87. doi: 10.1016/bs.vh.2018.01.010. Epub 2018 Feb 19. Vitam Horm. 2018. PMID: 29544643 Free PMC article. Review.
-
miR-122 Regulates LHR Expression in Rat Granulosa Cells by Targeting Insig1 mRNA.Endocrinology. 2018 May 1;159(5):2075-2082. doi: 10.1210/en.2017-03270. Endocrinology. 2018. PMID: 29579170 Free PMC article.
References
-
- LaPolt PS, Oikawa M, Jia XC, Dargan C, Hsueh AJ. Gonadotropin-induced up- and down-regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology. 1990;126:3277–3279 - PubMed
-
- Segaloff DL, Wang HY, Richards JS. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol. 1990;4:1856–1865 - PubMed
-
- Peegel H, Randolph J, Jr, Midgley AR, Menon KMJ. In situ hybridization of luteinizing hormone/human chorionic gonadotropin receptor messenger ribonucleic acid during hormone-induced down-regulation and the subsequent recovery in rat corpus luteum. Endocrinology. 1994;135:1044–1051 - PubMed
-
- Nair AK, Kash JC, Peegel H, Menon KMJ. Post-transcriptional regulation of luteinizing hormone receptor mRNA in the ovary by a novel mRNA-binding protein. J Biol Chem. 2002;277:21468–21473 - PubMed
-
- Hoffman YM, Peegel H, Sprock MJ, Zhang QY, Menon KMJ. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down-regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology. 1991;128:388–393 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

