CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis
- PMID: 24064383
- PMCID: PMC3859835
- DOI: 10.1016/j.freeradbiomed.2013.09.009
CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis
Abstract
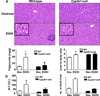
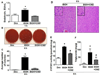
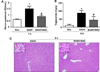
Ethanol-inducible cytochrome P450 2E1 (CYP2E1) contributes to increased oxidative stress and steatosis in chronic alcohol-exposure models. However, its role in binge ethanol-induced gut leakiness and hepatic injury is unclear. This study was aimed at investigating the role of CYP2E1 in binge alcohol-induced gut leakiness and the mechanisms of steatohepatitis. Female wild-type (WT) and Cyp2e1-null mice were treated with three doses of binge ethanol (WT-EtOH or Cyp2e1-null-EtOH) (6g/kg oral gavage at 12-h intervals) or dextrose (negative control). Intestinal histology of only WT-EtOH exhibited epithelial alteration and blebbing of lamina propria, and liver histology obtained at 6h after the last ethanol dose showed elevated steatosis with scattered inflammatory foci. These were accompanied by increased levels of serum endotoxin, hepatic enterobacteria, and triglycerides. All these changes, including the intestinal histology and hepatic apoptosis, determined by TUNEL assay, were significantly reversed when WT-EtOH mice were treated with the specific inhibitor of CYP2E1 chlormethiazole and the antioxidant N-acetylcysteine, both of which suppressed oxidative markers including intestinal CYP2E1. WT-EtOH also exhibited elevated amounts of serum TNF-α, hepatic cytokines, CYP2E1, and lipid peroxidation, with decreased levels of mitochondrial superoxide dismutase and suppressed aldehyde dehydrogenase 2 activity. Increased hepatocyte apoptosis with elevated levels of proapoptotic proteins and decreased levels of active (phosphorylated) p-AKT, p-AMPK, and peroxisome proliferator-activated receptor-α, all of which are involved in fat metabolism and inflammation, were observed in WT-EtOH. These changes were significantly attenuated in the corresponding Cyp2e1-null-EtOH mice. These data indicate that both intestinal and hepatic CYP2E1 induced by binge alcohol seems critical in binge alcohol-mediated increased nitroxidative stress, gut leakage, and endotoxemia; altered fat metabolism; and inflammation contributing to hepatic apoptosis and steatohepatitis.
Keywords: 3-NT; 3-nitrotyrosine; AFLD; ALD; ALDH2; ALT; ASH; Apoptosis; Binge ethanol; CMZ; CYP2E1; Cyp2e1-null–dextrose; Cyp2e1-null–ethanol; Free radicals; Gut leakiness; HAE; HPF; Liver; MDA; N-acetylcysteine; NAC; NAFLD; NASH; Oxidative stress; PPAR-α; RNS; ROS; SOD2; Steatohepatitis; TG; TNF-α; TUNEL; WT; WT-DEX; WT-EtOH; alanine aminotransferase; alcoholic fatty liver disease; alcoholic liver disease; alcoholic steatohepatitis; chlormethiazole; cytochrome P450 2E1; high-power field; hydroxyalkenal; malondialdehyde; mitochondrial aldehyde dehydrogenase 2; mitochondrial superoxide dismutase; nonalcoholic fatty liver disease; nonalcoholic steatohepatitis; null–DEX; null–EtOH; peroxisome proliferator-activated receptor-α; reactive nitrogen species; reactive oxygen species; terminal deoxynucleotidyl transferase dUTP nick-end labeling; triglyceride; tumor necrosis factor-α; wild-type; wild-type–dextrose; wild-type–ethanol..
Published by Elsevier Inc.
Conflict of interest statement
Figures




References
-
- Dufour MC, Stinson FS, Caces MF. Trends in cirrhosis morbidity and mortality: United States, 1979–1988. Semin. Liver. Dis. 1993;13:109–125. - PubMed
-
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N. Engl. J. Med. 2009;360:2758–2769. - PubMed
-
- Stickel F, Seitz HK. Alcoholic steatohepatitis. Best Pract. Res. Clin. Gastroenterol. 2010;24:683–693. - PubMed
-
- Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

