A modular approach to triazole-containing chemical inducers of dimerisation for yeast three-hybrid screening
- PMID: 24064457
- PMCID: PMC4031444
- DOI: 10.3390/molecules180911639
A modular approach to triazole-containing chemical inducers of dimerisation for yeast three-hybrid screening
Abstract
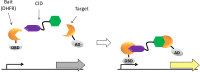
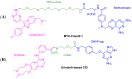
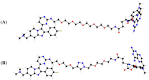
The yeast three-hybrid (Y3H) approach shows considerable promise for the unbiased identification of novel small molecule-protein interactions. In recent years, it has been successfully used to link a number of bioactive molecules to novel protein binding partners. However despite its potential importance as a protein target identification method, the Y3H technique has not yet been widely adopted, in part due to the challenges associated with the synthesis of the complex chemical inducers of dimerisation (CIDs). The development of a modular approach using potentially "off the shelf" synthetic components was achieved and allowed the synthesis of a family of four triazole-containing CIDs, MTX-Cmpd2.2-2.5. These CIDs were then compared using the Y3H approach with three of them giving a strong positive interaction with a known target of compound 2, TgCDPK1. These results showed that the modular nature of our synthetic strategy may help to overcome the challenges currently encountered with CID synthesis and should contribute to the Y3H approach reaching its full potential as an unbiased target identification strategy.
Figures



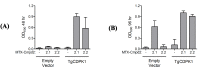


Similar articles
-
The yeast three-hybrid system as an experimental platform to identify proteins interacting with small signaling molecules in plant cells: potential and limitations.Front Plant Sci. 2011 Dec 26;2:101. doi: 10.3389/fpls.2011.00101. eCollection 2011. Front Plant Sci. 2011. PMID: 22639623 Free PMC article.
-
Facile synthesis of 1,2,3-triazole analogs of SGLT2 inhibitors by 'click chemistry'.Bioorg Med Chem Lett. 2012 Jan 1;22(1):642-4. doi: 10.1016/j.bmcl.2011.10.062. Epub 2011 Oct 24. Bioorg Med Chem Lett. 2012. PMID: 22079028
-
Facile synthesis of cids: biotinylated estrone oximes efficiently heterodimerize estrogen receptor and streptavidin proteins in yeast three hybrid systems.Org Lett. 2004 Apr 29;6(9):1409-12. doi: 10.1021/ol0497537. Org Lett. 2004. PMID: 15101754
-
Novel Hybrid Molecules Based on triazole-β-lactam as Potential Biological Agents.Mini Rev Med Chem. 2021;21(5):536-553. doi: 10.2174/1389557520666201027160436. Mini Rev Med Chem. 2021. PMID: 33109046 Review.
-
10 years of click chemistry: synthesis and applications of ferrocene-derived triazoles.Chem Asian J. 2011 Oct 4;6(10):2670-94. doi: 10.1002/asia.201100408. Epub 2011 Aug 31. Chem Asian J. 2011. PMID: 21882351 Review.
Cited by
-
Roles of DNA Target in Cancer Cell-Selective Cytotoxicity by Dicopper Complexes with DNA Target/Ligand Conjugates.ACS Omega. 2023 Jul 27;8(31):28690-28701. doi: 10.1021/acsomega.3c03387. eCollection 2023 Aug 8. ACS Omega. 2023. PMID: 37576680 Free PMC article.
-
Charged Triazole Cross-Linkers for Hyaluronan-Based Hybrid Hydrogels.Materials (Basel). 2016 Sep 30;9(10):810. doi: 10.3390/ma9100810. Materials (Basel). 2016. PMID: 28773931 Free PMC article.
-
Yeast three-hybrid screen identifies TgBRADIN/GRA24 as a negative regulator of Toxoplasma gondii bradyzoite differentiation.PLoS One. 2015 Mar 19;10(3):e0120331. doi: 10.1371/journal.pone.0120331. eCollection 2015. PLoS One. 2015. PMID: 25789621 Free PMC article.
-
Novel β-Cyclodextrin-Based Heptavalent Glycyrrhetinic Acid Conjugates: Synthesis, Characterization, and Anti-Influenza Activity.Front Chem. 2022 Apr 12;10:836955. doi: 10.3389/fchem.2022.836955. eCollection 2022. Front Chem. 2022. PMID: 35494649 Free PMC article.
-
Structure-activity relationship studies of lipophilic teicoplanin pseudoaglycon derivatives as new anti-influenza virus agents.Eur J Med Chem. 2018 Sep 5;157:1017-1030. doi: 10.1016/j.ejmech.2018.08.058. Epub 2018 Aug 22. Eur J Med Chem. 2018. PMID: 30170320 Free PMC article.
References
-
- Becker F., Murthi K., Smith C., Come J., Costa-Roldán N., Kaufmann C., Hanke U., Degenhart C., Baumann S., Wallner W., et al. A Three-Hybrid Approach to Scanning the Proteome for Targets of Small Molecule Kinase Inhibitors. Chem. Biol. 2004;11:211–223. - PubMed
-
- Caligiuri M., Becker F., Murthi K., Kaplan F., Dedier S., Kaufmann C., Machl A., Zybarth G., Richard J., Bockovich N., Kluge A., Kley N. A Proteome-Wide CDK/CRK-Specific Kinase Inhibitor Promotes Tumor Cell Death in the Absence of Cell Cycle Progression. Chem. Biol. 2005;12:1103–1115. doi: 10.1016/j.chembiol.2005.08.008. - DOI - PubMed
-
- Caligiuri M., Molz L., Liu Q., Kaplan F., Xu J.P., Majeti J.Z., Ramos-Kelsey R., Murthi K., Lievens S., Tavernier J., Kley N. MASPIT: Three-Hybrid Trap for Quantitative Proteome Fingerprinting of Small Molecule-Protein Interactions in Mammalian Cells. Chem. Biol. 2006;13:711–722. doi: 10.1016/j.chembiol.2006.05.008. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

