New β-phospholactam as a carbapenem transition state analog: Synthesis of a broad-spectrum inhibitor of metallo-β-lactamases
- PMID: 24064498
- PMCID: PMC3833270
- DOI: 10.1016/j.bmcl.2013.08.098
New β-phospholactam as a carbapenem transition state analog: Synthesis of a broad-spectrum inhibitor of metallo-β-lactamases
Abstract
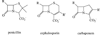
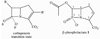
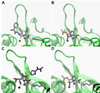
In an effort to test whether a transition state analog is an inhibitor of the metallo-β-lactamases, a phospholactam analog of carbapenem has been synthesized and characterized. The phospholactam 1 proved to be a weak, time-dependent inhibitor of IMP-1 (70%), CcrA (70%), L1 (70%), NDM-1 (53%), and Bla2 (94%) at an inhibitor concentration of 100μM. The phospholactam 1 activated ImiS and BcII at the same concentration. Docking studies were used to explain binding and to offer suggestions for modifications to the phospholactam scaffold to improve binding affinities.
Keywords: Inhibitor; Metallo-β-lactamase; Phospholactam.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
High prevalence of metallo-β-lactamase among carbapenem-resistant Klebsiella pneumoniae in a teaching hospital in China.Can J Microbiol. 2014 Oct;60(10):691-5. doi: 10.1139/cjm-2014-0291. Epub 2014 Sep 8. Can J Microbiol. 2014. PMID: 25285938
-
Detection of carbapenem-resistant Escherichia coli and Klebsiella pneumoniae producing NDM-1 in Lebanon.J Infect Dev Ctries. 2012 May 14;6(5):457-61. doi: 10.3855/jidc.2340. J Infect Dev Ctries. 2012. PMID: 22610714
-
Pterostilbene restores carbapenem susceptibility in New Delhi metallo-β-lactamase-producing isolates by inhibiting the activity of New Delhi metallo-β-lactamases.Br J Pharmacol. 2019 Dec;176(23):4548-4557. doi: 10.1111/bph.14818. Epub 2019 Dec 9. Br J Pharmacol. 2019. PMID: 31376166 Free PMC article.
-
Ten Years with New Delhi Metallo-β-lactamase-1 (NDM-1): From Structural Insights to Inhibitor Design.ACS Infect Dis. 2019 Jan 11;5(1):9-34. doi: 10.1021/acsinfecdis.8b00247. Epub 2018 Nov 28. ACS Infect Dis. 2019. PMID: 30421910 Review.
-
New Carbapenemase Inhibitors: Clearing the Way for the β-Lactams.Int J Mol Sci. 2020 Dec 6;21(23):9308. doi: 10.3390/ijms21239308. Int J Mol Sci. 2020. PMID: 33291334 Free PMC article. Review.
Cited by
-
Targeting Metalloenzymes for Therapeutic Intervention.Chem Rev. 2019 Jan 23;119(2):1323-1455. doi: 10.1021/acs.chemrev.8b00201. Epub 2018 Sep 7. Chem Rev. 2019. PMID: 30192523 Free PMC article. Review.
-
Metallo-β-Lactamase Inhibitors Inspired on Snapshots from the Catalytic Mechanism.Biomolecules. 2020 Jun 3;10(6):854. doi: 10.3390/biom10060854. Biomolecules. 2020. PMID: 32503337 Free PMC article. Review.
-
Metallo-β-lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design.Chem Rev. 2021 Jul 14;121(13):7957-8094. doi: 10.1021/acs.chemrev.1c00138. Epub 2021 Jun 15. Chem Rev. 2021. PMID: 34129337 Free PMC article. Review.
-
Cyclobutanone Mimics of Intermediates in Metallo-β-Lactamase Catalysis.Chemistry. 2018 Apr 17;24(22):5734-5737. doi: 10.1002/chem.201705886. Epub 2018 Jan 17. Chemistry. 2018. PMID: 29250863 Free PMC article.
-
Dynamically chiral phosphonic acid-type metallo-β-lactamase inhibitors.Commun Chem. 2025 Apr 19;8(1):119. doi: 10.1038/s42004-025-01510-5. Commun Chem. 2025. PMID: 40253435 Free PMC article.
References
-
- Mitcher LA, Lemke TL, Gentry EJ. In: Foye's Principles of Medicinal Chemistry. 6th ed. Lemke TL, Williams DA, Roche VF, Zito SW, editors. Wolters Kluwer Lippincott Williams & Wilkins; 2007.
-
- Bush K. Curr. Opin. Pharmacol. 2012;12:527. - PubMed
-
- Bush K, Macielag MJ. Expert Opin. Thera. Patents. 2010;20:1277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

