A randomized, placebo-controlled phase I study assessing the safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein OprF/I vaccine (IC43) in healthy volunteers
- PMID: 24064511
- PMCID: PMC4181012
- DOI: 10.4161/hv.26565
A randomized, placebo-controlled phase I study assessing the safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein OprF/I vaccine (IC43) in healthy volunteers
Abstract
Introduction: IC43 is a recombinant outer membrane protein-based vaccine against Pseudomonas aeruginosa (P. aeruginosa) consisting of OprF- and OprI- epitopes (Opr, outer membrane protein; OprF/I, OprF/OprI hybrid vaccine) with an N-terminal His 6 tag (Met-Ala-(His)6-OprF190-342-OprI21-83).
Objectives: The study aimed to confirm the optimal dose of IC43 in adults with regard to immunogenicity, safety, and tolerability after vaccination with three different dosages of IC43, compared with placebo, and to investigate a potential immune-enhancing effect of the adjuvant, aluminum hydroxide. Subjects were randomly allocated in a 1:1:1:1:1 ratio to one of five treatment groups: 50, 100, or 200 µg IC43 with adjuvant, 100 µg IC43 without adjuvant, or placebo (0.9% sodium chloride) and two intramuscular injections were given in the deltoid region 7 d apart.
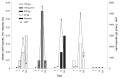
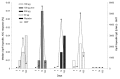
Results: The primary immunogenicity analysis of OprF/I-specific IgG antibody titers on day 14 demonstrated statistically significant differences among treatment groups (P<0.0001), with a significantly higher immune response detected in each IC43 treatment group compared with placebo. From day 0 to day 14, a ≥4-fold increase in OprF/I-specific immunoglobulin G (IgG) antibody titers were observed in>90% of subjects in all IC43 treatment groups in the per-protocol (PP) and intention-to-treat (ITT) populations; a ≥50-fold titer increase was observed in 42.6% subjects including all IC43 treatment groups. On day 90, OprF/I-specific IgGs started to decline in all IC43 treatment groups but remained significantly higher until 6 mo compared with placebo. Assessment of functional antibody induction by opsonophagocytic assay (OPA) followed a similar pattern compared with OprF/I-specific IgG kinetics. At day 14, a ≥2-fold increase in OPA titer was observed in 54.5% subjects within all IC43 treatment groups. An increase in antibody avidity index was observed after the second vaccination. At day 14, >96% of subjects in each IC43 treatment group had detectable OprF/I-specific IgG antibodies. Anti-histidine IgG antibody titers peaked on day 14 and were reduced on day 90 in all IC43 treatment groups. OprF/I-specific IgG secreted by antibody-secreting cell (ASC) was detected in all IC43 groups by B-cell ELIspot after the second vaccination and up to 6 mo. All vaccinations were safe and well tolerated up to the maximum cumulative dosage of 400 µg IC43.
Conclusion: IC43 doses equal to or greater than 50 µg were sufficient to induce a plateau of IgG antibody responses in healthy volunteers. Higher doses, whether adjuvanted or non-adjuvanted, were not more effective.
Methods: In this phase I, randomized, placebo-controlled, observer-blinded, multicenter clinical trial, 163 healthy volunteers (18-65 y) were randomly assigned to five treatment groups (1:1:1:1:1). Three groups received IC43 with adjuvant: 50 µg (n=32), 100 µg (n=33), or 200 µg (n=33). One group received IC43 100 µg without adjuvant (n=32), and one group received placebo (0.9% sodium chloride) (n=33). Each subject received two intramuscular vaccinations, separated by a 7-d interval (days 0 and 7) (Fig. 1). Humoral immune response was assessed by measurement of outer membrane protein F/I (OprF/I)-specific antibodies determined by enzyme-linked immunosorbent assay (ELISA), anti-histidine antibodies determined by ELISA, and functional antibody activity determined by opsonophagocytic assay (OPA), up to 6 mo post-vaccination. Antibody avidity was measured on days 7 and 14 from samples that had detectable vaccine antibody-specific immunoglobulin G (IgG) antibody titers. At the Austrian site only, the B-cell ELIspot assay was used to determine specific ASC responses. Safety was assessed using adverse event monitoring and clinical laboratory tests. Local and systemic tolerability was recorded in a subject diary for 7 d after each vaccination and by investigators up to 6 mo post-vaccination.
Keywords: adjuvant; bacterial infection; dose-response relationship; immunity; vaccination.
Figures

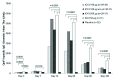
Similar articles
-
A randomized placebo-controlled phase II study of a Pseudomonas vaccine in ventilated ICU patients.Crit Care. 2017 Feb 4;21(1):22. doi: 10.1186/s13054-017-1601-9. Crit Care. 2017. PMID: 28159015 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers.Infect Immun. 1999 Mar;67(3):1461-70. doi: 10.1128/IAI.67.3.1461-1470.1999. Infect Immun. 1999. PMID: 10024596 Free PMC article.
-
Immunization with outer membrane proteins (OprF and OprI) and flagellin B protects mice from pulmonary infection with mucoid and nonmucoid Pseudomonas aeruginosa.J Microbiol Immunol Infect. 2018 Jun;51(3):312-320. doi: 10.1016/j.jmii.2016.08.014. Epub 2017 Feb 20. J Microbiol Immunol Infect. 2018. PMID: 28291719
-
Immunogenicity and safety of adjuvant-associated COVID-19 vaccines: A systematic review and meta-analysis of randomized controlled trials.Heliyon. 2023 Nov 28;9(12):e22858. doi: 10.1016/j.heliyon.2023.e22858. eCollection 2023 Dec. Heliyon. 2023. PMID: 38125524 Free PMC article. Review.
-
COVID-19 vaccination effectiveness and safety in vulnerable populations: a meta-analysis of 33 observational studies.Front Pharmacol. 2023 Jun 23;14:1144824. doi: 10.3389/fphar.2023.1144824. eCollection 2023. Front Pharmacol. 2023. PMID: 37426814 Free PMC article.
Cited by
-
Identification of OprF as a complement component C3 binding acceptor molecule on the surface of Pseudomonas aeruginosa.Infect Immun. 2015 Aug;83(8):3006-14. doi: 10.1128/IAI.00081-15. Epub 2015 May 11. Infect Immun. 2015. PMID: 25964476 Free PMC article.
-
Genome-Based Approach Delivers Vaccine Candidates Against Pseudomonas aeruginosa.Front Immunol. 2019 Jan 9;9:3021. doi: 10.3389/fimmu.2018.03021. eCollection 2018. Front Immunol. 2019. PMID: 30687303 Free PMC article.
-
PA0833 Is an OmpA C-Like Protein That Confers Protection Against Pseudomonas aeruginosa Infection.Front Microbiol. 2018 May 23;9:1062. doi: 10.3389/fmicb.2018.01062. eCollection 2018. Front Microbiol. 2018. PMID: 29875759 Free PMC article.
-
Vaccination to Prevent Pseudomonas aeruginosa Bloodstream Infections.Front Microbiol. 2022 Mar 28;13:870104. doi: 10.3389/fmicb.2022.870104. eCollection 2022. Front Microbiol. 2022. PMID: 35418967 Free PMC article.
-
Bioengineering a bacterial pathogen to assemble its own particulate vaccine capable of inducing cellular immunity.Sci Rep. 2017 Feb 2;7:41607. doi: 10.1038/srep41607. Sci Rep. 2017. PMID: 28150705 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
