Functions and mechanics of dynein motor proteins
- PMID: 24064538
- PMCID: PMC3972880
- DOI: 10.1038/nrm3667
Functions and mechanics of dynein motor proteins
Abstract
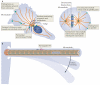
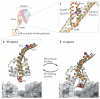
Fuelled by ATP hydrolysis, dyneins generate force and movement on microtubules in a wealth of biological processes, including ciliary beating, cell division and intracellular transport. The large mass and complexity of dynein motors have made elucidating their mechanisms a sizable task. Yet, through a combination of approaches, including X-ray crystallography, cryo-electron microscopy, single-molecule assays and biochemical experiments, important progress has been made towards understanding how these giant motor proteins work. From these studies, a model for the mechanochemical cycle of dynein is emerging, in which nucleotide-driven flexing motions within the AAA+ ring of dynein alter the affinity of its microtubule-binding stalk and reshape its mechanical element to generate movement.
Figures



References
-
- Moughamian AJ, Holzbaur E. G protein-related fold. In: King SM, editor. Dyneins: Structure, Biology and Disease. Elsevier Inc.; 2011. pp. 584–601.
-
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. - PubMed
-
- Gibbons I, Rowe A. Dynein: a protein with adenosine triphosphatase activity from cilia. Science. 1965;149:424–426. [References 3 and 4 mark the discovery of dynein motor proteins.] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 092436/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- BB/E00928X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K000705/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 092436/WT_/Wellcome Trust/United Kingdom
- BB/BB/K000705/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

