Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity
- PMID: 24064762
- PMCID: PMC6595219
- DOI: 10.1038/ncomms3384
Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity
Abstract
The antioxidant tempol reduces obesity in mice. Here we show that tempol alters the gut microbiome by preferentially reducing the genus Lactobacillus and its bile salt hydrolase (BSH) activity leading to the accumulation of intestinal tauro-β-muricholic acid (T-β-MCA). T-β-MCA is an farnesoid X receptor (FXR) nuclear receptor antagonist, which is involved in the regulation of bile acid, lipid and glucose metabolism. Its increased levels during tempol treatment inhibit FXR signalling in the intestine. High-fat diet-fed intestine-specific Fxr-null (Fxr(ΔIE)) mice show lower diet-induced obesity, similar to tempol-treated wild-type mice. Further, tempol treatment does not decrease weight gain in Fxr(ΔIE) mice, suggesting that the intestinal FXR mediates the anti-obesity effects of tempol. These studies demonstrate a biochemical link between the microbiome, nuclear receptor signalling and metabolic disorders, and suggest that inhibition of FXR in the intestine could be a target for anti-obesity drugs.
Conflict of interest statement
Figures

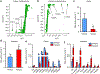
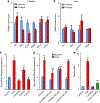
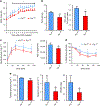


References
-
- Khandekar MJ, Cohen P & Spiegelman BM Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 11, 886–895 (2011). - PubMed
-
- Li Y et al. Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes 57, 817–827 (2008). - PubMed
-
- Wadikar DD & Premavalli KS Appetite control and obesity. Crit. Rev. Food. Sci. Nutr 52, 949–956 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

