Thrombocytopenia in pegylated interferon and ribavirin combination therapy for chronic hepatitis C
- PMID: 24065124
- PMCID: PMC4124258
- DOI: 10.1007/s00535-013-0884-0
Thrombocytopenia in pegylated interferon and ribavirin combination therapy for chronic hepatitis C
Abstract
Background: This study aimed to examine the therapeutic effect and prognostic indicators of pegylated interferon (PEG-IFN) and ribavirin (RBV) combination therapy in thrombocytopenic patients with chronic hepatitis C, hepatitis C virus (HCV)-related cirrhosis, and those who underwent splenectomy or partial splenic embolization (PSE).
Methods: Of 326 patients with HCV-related chronic liver disease (252 with genotype 1b and 74 with genotype 2a/2b) treated with PEG-IFN/RBV, 90 were diagnosed with cirrhosis.
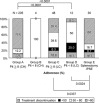
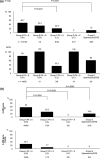
Results: Regardless of the degree of thrombocytopenia, the administration rate was significantly higher in the splenectomy/PSE group compared to the cirrhosis group. However, in patients with genotype 1b, the sustained virological response (SVR) rate was significantly lower in the cirrhosis and the splenectomy/PSE groups compared to the chronic hepatitis group. No cirrhotic patients with platelets less than 80,000 achieved an SVR. Patients with genotype 2a/2b were more likely to achieve an SVR than genotype 1b. Prognostic factors for SVR in patients with genotype 1b included the absence of esophageal and gastric varices, high serum ALT, low AST/ALT ratio, and the major homo type of the IL28B gene. Splenectomy- or PSE-facilitated induction of IFN in patients with genotype 2a/2b was more likely to achieve an SVR by an IFN dose maintenance regimen. Patients with genotype 1b have a low SVR regardless of splenectomy/PSE. In particular, patients with a hetero/minor type of IL28B did not have an SVR.
Conclusions: Splenectomy/PSE for IFN therapy should be performed in patients expected to achieve a treatment response, considering their genotype and IL28B.
Figures
Similar articles
-
Effect of spleen operation on antiviral treatment in hepatitis C virus-related cirrhotic patients.World J Gastroenterol. 2014 Nov 7;20(41):15387-97. doi: 10.3748/wjg.v20.i41.15387. World J Gastroenterol. 2014. PMID: 25386089 Free PMC article.
-
Effect of laparoscopic splenectomy in patients with Hepatitis C and cirrhosis carrying IL28B minor genotype.BMC Gastroenterol. 2012 Nov 12;12:158. doi: 10.1186/1471-230X-12-158. BMC Gastroenterol. 2012. PMID: 23145809 Free PMC article.
-
Combination therapy of low-dose of PegIFNa-2a and ribavirin for patients with HCV-infected decompensated cirrhosis.Hepatogastroenterology. 2013 Sep;60(126):1391-8. doi: 10.5754/hge121194. Hepatogastroenterology. 2013. PMID: 23635509
-
Sustained virologic response and IL28B single-nucleotide polymorphisms in patients with chronic hepatitis C treated with pegylated interferon alfa and ribavirin.Acta Biochim Pol. 2012;59(3):333-7. Epub 2012 Aug 27. Acta Biochim Pol. 2012. PMID: 22924160
-
Treatment of chronic hepatitis C in southern Taiwan.Intervirology. 2006;49(1-2):99-106. doi: 10.1159/000087271. Intervirology. 2006. PMID: 16166797 Review.
Cited by
-
Possible Relevance of PNPLA3 and TLL1 Gene Polymorphisms to the Efficacy of PEG-IFN Therapy for HBV-Infected Patients.Int J Mol Sci. 2020 Apr 27;21(9):3089. doi: 10.3390/ijms21093089. Int J Mol Sci. 2020. PMID: 32349377 Free PMC article.
-
Effects of Helicobacter pylori Eradication on the Platelet Count in Hepatitis C Virus-Infected Patients.J Clin Med Res. 2016 Dec;8(12):854-858. doi: 10.14740/jocmr2725w. Epub 2016 Oct 26. J Clin Med Res. 2016. PMID: 27829950 Free PMC article.
-
Effect of spleen operation on antiviral treatment in hepatitis C virus-related cirrhotic patients.World J Gastroenterol. 2014 Nov 7;20(41):15387-97. doi: 10.3748/wjg.v20.i41.15387. World J Gastroenterol. 2014. PMID: 25386089 Free PMC article.
-
Impact of Sustained Virological Response for Gastroesophageal Varices in Hepatitis-C-Virus-Related Liver Cirrhosis.J Clin Med. 2019 Dec 30;9(1):95. doi: 10.3390/jcm9010095. J Clin Med. 2019. PMID: 31905953 Free PMC article.
References
-
- Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. - DOI - PubMed
-
- Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HY, Sterling RK, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840–849. doi: 10.1053/j.gastro.2010.11.050. - DOI - PMC - PubMed
-
- Fernández-Rodríguez CM, Alonso S, Martinez SM, Forns X, Sanchez-Tapias JM, Rincón D, et al. Peginterferon plus ribavirin and sustained virological response in HCV-related cirrhosis: outcomes and factors predicting response. Am J Gastroenterol. 2010;105:2164–2172. doi: 10.1038/ajg.2010.294. - DOI - PubMed
-
- Qu LS, Chen H, Kuai XL, Xu ZF, Jin F, Zhou GX. Effects of interferon therapy on development of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis: a meta-analysis of randomized controlled trials. Hepatol Res. 2012;42:782–789. doi: 10.1111/j.1872-034X.2012.00984.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

