Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis
- PMID: 24065148
- PMCID: PMC7107356
- DOI: 10.1093/infdis/jit504
Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis
Abstract
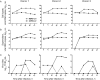
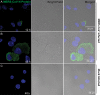
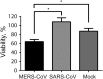
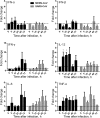
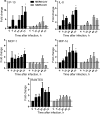
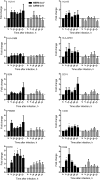
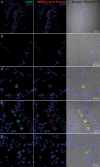
Middle East respiratory syndrome coronavirus (MERS-CoV) infection caused severe pneumonia and multiorgan dysfunction and had a higher crude fatality rate (around 50% vs. 10%) than SARS coronavirus (SARS-CoV) infection. To understand the pathogenesis, we studied viral replication, cytokine/chemokine response, and antigen presentation in MERS-CoV-infected human monocyte-derived macrophages (MDMs) versus SARS-CoV-infected MDMs. Only MERS-CoV can replicate in MDMs. Both viruses were unable to significantly stimulate the expression of antiviral cytokines (interferon α [IFN-α] and IFN-β) but induced comparable levels of tumor necrosis factor α and interleukin 6. Notably, MERS-CoV induced significantly higher expression levels of interleukin 12, IFN-γ, and chemokines (IP-10/CXCL-10, MCP-1/CCL-2, MIP-1α/CCL-3, RANTES/CCL-5, and interleukin 8) than SARS-CoV. The expression of major histocompatibility complex class I and costimulatory molecules were significantly higher in MERS-CoV-infected MDMs than in SARS-CoV-infected cells. MERS-CoV replication was validated by immunostaining of infected MDMs and ex vivo lung tissue. We conclusively showed that MERS-CoV can establish a productive infection in human macrophages. The aberrant induction of inflammatory cytokines/chemokines could be important in the disease pathogenesis.
Keywords: MERS-CoV; SARS-CoV; cytokine and chemokine response; pathogenesis; viral replication.
Figures
References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. - PubMed
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV)—update. 1 August 2013. http://www.who.int/csr/don/2013_08_01/en/index.html . Accessed 27 September 2013.
-
- Woo PC, Lau SK, Huang Y, Yuen KY. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med (Maywood) 2009;234:1117–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

