Independent skewing of the T cell and NK cell compartments associated with cytomegalovirus infection suggests division of labor between innate and adaptive immunity
- PMID: 24065293
- PMCID: PMC4039248
- DOI: 10.1007/s11357-013-9587-y
Independent skewing of the T cell and NK cell compartments associated with cytomegalovirus infection suggests division of labor between innate and adaptive immunity
Abstract
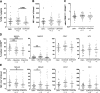
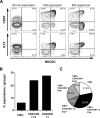
Cytomegalovirus (CMV) infection induces profound changes in different subsets of the cellular immune system. We have previously identified an immune risk profile (IRP) where CMV-associated changes in the T cell compartment, defined as a CD4/CD8 ratio < 1, are associated with increased mortality in elderly people. Since natural killer (NK) cells have an important role in the defense against viral infections, we examined whether the expansion of CD8 + T cells seen in individuals with CD4/CD8 ratio < 1 is coupled to a parallel skewing of the NK cell compartment. A number of 151 subjects were examined with CMV serology and a flow cytometry panel for assessment of T cell and NK cell subsets. CMV-seropositive individuals had higher frequencies of CD57 + and NKG2C + NK cells and lower frequencies of NKG2A + NK cells, in line with a more differentiated NK cell compartment. Intriguingly, however, there was no correlation between CD4/CD8 ratio and NK cell repertoires among CMV-seropositive donors, despite the profound skewing of the T cell compartment in the group with CD4/CD8 ratio < 1. Conversely, donors with profound expansion of NK cells, defined as NKG2C + NK cells with high expression of CD57 and ILT-2, did not display more common changes in their T cell repertoire, suggesting that NK cell expansion is independent of the T cell-defined IRP. Altogether, these results indicate that the effect of CMV on CD8 T cells and NK cells is largely nonoverlapping and independent.
Figures



References
-
- Beziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debre P, Bjorkstrom NK, Malmberg KJ, Marcellin P, Vieillard V. CMV drives clonal expansion of NKG2C + NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42(2):447–457. doi: 10.1002/eji.201141826. - DOI - PubMed
-
- Beziat V, Liu L, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT, Retiere C, Sverremark-Ekstrom E, Traherne J, Ljungman P, Schaffer M, Price DA, Trowsdale J, Michaelsson J, Ljunggren HG, Malmberg KJ. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121(14):2678–2688. doi: 10.1182/blood-2012-10-459545. - DOI - PMC - PubMed
-
- Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaelsson J, Malmberg KJ, Klingstrom J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. doi: 10.1084/jem.20100762. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
