Evaluation of YO-PRO-1 as an early marker of apoptosis following radiofrequency ablation of colon cancer liver metastases
- PMID: 24065619
- PMCID: PMC3918265
- DOI: 10.1007/s10616-013-9565-3
Evaluation of YO-PRO-1 as an early marker of apoptosis following radiofrequency ablation of colon cancer liver metastases
Abstract
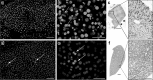
Radiofrequency (RF) ablation (RFA) is a minimally invasive treatment for colorectal-cancer liver metastases (CLM) in selected nonsurgical patients. Unlike surgical resection, RFA is not followed by routine pathological examination of the target tumor and the surrounding liver tissue. The aim of this study was the evaluation of apoptotic events after RFA. Specifically, we evaluated YO-PRO-1 (YP1), a green fluorescent DNA marker for cells with compromised plasma membrane, as a potential, early marker of cell death. YP1 was applied on liver tissue adherent on the RF electrode used for CLM ablation, as well as on biopsy samples from the center and the margin of the ablation zone as depicted by dynamic CT immediately after RFA. Normal pig and mouse liver tissues were used for comparison. The same samples were also immunostained for fragmented DNA (TUNEL assay) and for active mitochondria (anti-OxPhos antibody). YP1 was also used simultaneously with propidium iodine (PI) to stain mouse liver and samples from ablated CLM. Following RFA of human CLM, more than 90 % of cells were positive for YP1. In nonablated, dissected pig and mouse liver however, we found similar YP1 signals (93.1 % and 65 %, respectively). In samples of intact mouse liver parenchyma, there was a significantly smaller proportion of YP1 positive cells (22.7 %). YP1 and PI staining was similar for ablated CLM. However in dissected normal mouse liver there was initial YP1 positivity and complete absence of the PI signal and only later there was PI signal.
Conclusion: This is the first time that YP1 was applied in liver parenchymal tissue (rather than cell culture). The results suggest that YP1 is a very sensitive marker of early cellular events reflecting an early and widespread plasma membrane injury that allows YP1 penetration into the cells.
Figures




References
-
- Boffa DJ, Waka J, Thomas D, Suh S, Curran K, Sharma VK, Besada M, Muthukumar T, Yang H, Suthanthiran M, Manova K. Measurement of apoptosis of intact human islets by confocal optical sectioning and stereologic analysis of YO-PRO-1-stained islets. Transplantation. 2005;79:842–845. doi: 10.1097/01.TP.0000155175.24802.73. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

