Forkhead box O1 is a repressor of basal and GnRH-induced Fshb transcription in gonadotropes
- PMID: 24065703
- PMCID: PMC3805845
- DOI: 10.1210/me.2013-1185
Forkhead box O1 is a repressor of basal and GnRH-induced Fshb transcription in gonadotropes
Abstract
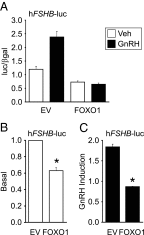
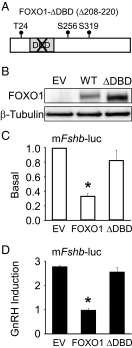
Synthesis of the gonadotropin β-subunits is tightly controlled by a complex network of hormonal signaling pathways that may be modulated by metabolic cues. Recently, we reported that insulin regulates FOXO1 phosphorylation and cellular localization in pituitary gonadotropes and that FOXO1 overexpression inhibits Lhb transcription. In the current study, we investigated whether FOXO1 modulates Fshb synthesis. Here, we demonstrate that FOXO1 represses basal and GnRH-induced Fshb transcription in LβT2 cells. In addition, we show that PI3K inhibition, which increases FOXO1 nuclear localization, results in decreased Fshb mRNA levels in murine primary pituitary cells. FOXO1 also decreases transcription from the human FSHB promoter, suggesting that FOXO1 regulation of FSHB transcription may be conserved between rodents and humans. Although the FOXO1 DNA-binding domain is necessary for suppression of Fshb, we do not observe direct binding of FOXO1 to the Fshb promoter, suggesting that FOXO1 exerts its effect through protein-protein interactions with transcription factors required for Fshb synthesis. FOXO1 suppression of basal Fshb transcription may involve PITX1 because PITX1 interacts with FOXO1, FOXO1 repression maps to the proximal Fshb promoter containing a PITX1-binding site, PITX1 induction of Fshb or a PITX1 binding element in CV-1 cells is decreased by FOXO1, and FOXO1 suppresses Pitx1 mRNA and protein levels. GnRH induction of an Fshb promoter containing a deletion at -50/-41 or -30/-21 is not repressed by FOXO1, suggesting that these two regions may be involved in FOXO1 suppression of GnRH-induced Fshb synthesis. In summary, our data demonstrate that FOXO1 can negatively regulate Fshb transcription and suggest that FOXO1 may relay metabolic hormonal signals to modulate gonadotropin production.
Figures






Similar articles
-
Constitutively active FOXO1 diminishes activin induction of Fshb transcription in immortalized gonadotropes.PLoS One. 2014 Nov 25;9(11):e113839. doi: 10.1371/journal.pone.0113839. eCollection 2014. PLoS One. 2014. PMID: 25423188 Free PMC article.
-
FOXO1 transcription factor inhibits luteinizing hormone β gene expression in pituitary gonadotrope cells.J Biol Chem. 2012 Sep 28;287(40):33424-35. doi: 10.1074/jbc.M112.362103. Epub 2012 Aug 3. J Biol Chem. 2012. PMID: 22865884 Free PMC article.
-
Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-beta gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1.Mol Endocrinol. 2011 Aug;25(8):1387-403. doi: 10.1210/me.2011-0032. Epub 2011 Jun 9. Mol Endocrinol. 2011. PMID: 21659477 Free PMC article.
-
Welcoming beta-catenin to the gonadotropin-releasing hormone transcriptional network in gonadotropes.Mol Endocrinol. 2008 Jun;22(6):1295-303. doi: 10.1210/me.2007-0515. Epub 2008 Jan 24. Mol Endocrinol. 2008. PMID: 18218726 Free PMC article. Review.
-
The nature of FSH induction by GnRH.Trends Endocrinol Metab. 2002 Aug;13(6):257-63. doi: 10.1016/s1043-2760(02)00614-8. Trends Endocrinol Metab. 2002. PMID: 12128287 Review.
Cited by
-
Acne Transcriptomics: Fundamentals of Acne Pathogenesis and Isotretinoin Treatment.Cells. 2023 Nov 10;12(22):2600. doi: 10.3390/cells12222600. Cells. 2023. PMID: 37998335 Free PMC article. Review.
-
Linking diet to acne metabolomics, inflammation, and comedogenesis: an update.Clin Cosmet Investig Dermatol. 2015 Jul 15;8:371-88. doi: 10.2147/CCID.S69135. eCollection 2015. Clin Cosmet Investig Dermatol. 2015. PMID: 26203267 Free PMC article. Review.
-
Difference in miRNA Expression in Functioning and Silent Corticotroph Pituitary Adenomas Indicates the Role of miRNA in the Regulation of Corticosteroid Receptors.Int J Mol Sci. 2022 Mar 5;23(5):2867. doi: 10.3390/ijms23052867. Int J Mol Sci. 2022. PMID: 35270010 Free PMC article.
-
FOXO Transcription Factors Are Required for Normal Somatotrope Function and Growth.Endocrinology. 2022 Feb 1;163(2):bqab263. doi: 10.1210/endocr/bqab263. Endocrinology. 2022. PMID: 34971379 Free PMC article.
-
SEMA6D Expression and Patient Survival in Breast Invasive Carcinoma.Int J Breast Cancer. 2015;2015:539721. doi: 10.1155/2015/539721. Epub 2015 Apr 20. Int J Breast Cancer. 2015. PMID: 25973277 Free PMC article.
References
-
- Apter D. Development of the hypothalamic-pituitary-ovarian axis. Ann N Y Acad Sci. 1997;816:9–21 - PubMed
-
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22(6):764–786 - PubMed
-
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204 - PubMed
-
- Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I. Human FSHβ subunit gene is highly conserved. Mol Hum Reprod. 2005;11(8):601–605 - PubMed
-
- Ortolano GA, Haisenleder DJ, Dalkin AC, et al. Follicle-stimulating hormone β subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology. 1988;123(6):2946–2948 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

