Regulation of hypothalamic corticotropin-releasing hormone transcription by elevated glucocorticoids
- PMID: 24065704
- PMCID: PMC3805848
- DOI: 10.1210/me.2013-1095
Regulation of hypothalamic corticotropin-releasing hormone transcription by elevated glucocorticoids
Abstract
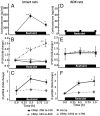
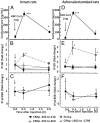
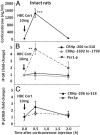
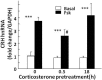
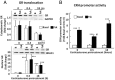
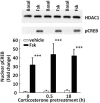
Negative glucocorticoid feedback is essential for preventing the deleterious effects of excessive hypothalamic pituitary adrenal axis axis activation, with an important target being CRH transcription in the hypothalamic paraventricular nucleus. The aim of these studies was to determine whether glucocorticoids repress CRH transcription directly in CRH neurons, by examining glucocorticoid effects on glucocorticoid receptor (GR)-CRH promoter interaction and the activation of proteins required for CRH transcription. Immunoprecipitation of hypothalamic chromatin from intact or adrenalectomized rats subjected to either stress or corticosterone injections showed minor association of the proximal CRH promoter with the GR compared with that with phospho-CREB (pCREB). In contrast, the Period-1 (Per1, a glucocorticoid-responsive gene) promoter markedly recruited GR. Stress increased pCREB recruitment by the CRH but not the Per1 promoter, irrespective of circulating glucocorticoids. In vitro, corticosterone pretreatment (30 minutes or 18 hours) only slightly inhibited basal and forskolin-stimulated CRH heteronuclear RNA in primary hypothalamic neuronal cultures and CRH promoter activity in hypothalamic 4B cells. In 4B cells, 30 minutes or 18 hours of corticosterone exposure had no effect on forskolin-induced nuclear accumulation of the recognized CRH transcriptional regulators, pCREB and transducer of regulated CREB activity 2. The data show that inhibition of CRH transcription by physiological glucocorticoids in vitro is minor and that direct interaction of GR with DNA in the proximal CRH promoter may not be a major mechanism of CRH gene repression. Although GR interaction with distal promoter elements may have a role, the data suggest that transcriptional repression of CRH by glucocorticoids involves protein-protein interactions and/or modulation of afferent inputs to the hypothalamic paraventricular nucleus.
Figures

References
-
- Antoni FA. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev. 1986;7:351–378 - PubMed
-
- Claes S. Corticotropin-releasing hormone (CRH) in psychiatry: from stress to psychopathology. Ann Med (Helsinki). 2004;36:50–61 - PubMed
-
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179 - PubMed
-
- Munck A, Náray-Fejes-Tóth A. Glucocorticoids and stress: permissive and suppressive actions. Ann NY Acad Sci. 1994;746:115–130; discussion 131–133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

