Flow cytometric immunophenotyping of feline bone marrow cells and haematopoietic progenitor cells using anti-human antibodies
- PMID: 24065708
- PMCID: PMC11383116
- DOI: 10.1177/1098612X13505575
Flow cytometric immunophenotyping of feline bone marrow cells and haematopoietic progenitor cells using anti-human antibodies
Abstract
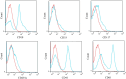
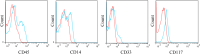
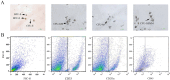
There is a paucity of species-specific antibodies available for feline haematopoietic conditions. The purpose of this study was to broaden the panel of antibodies available for use in the immunophenotypic characterisation of feline haematopoietic cells by testing clones of anti-human monoclonal antibodies (mAbs) on normal, neoplastic and cultured feline haematopoietic progenitors to determine cross-reactivity to feline counterparts. In this study, 24 clones of anti-human mAbs were tested on normal or neoplastic feline bone marrow and peripheral blood cells. Six of these mAbs, including anti-cluster of differentiation (CD)61, anti-CD18, anti-CD14, anti-CD235a, anti-CD41 and anti-CD29, cross-reacted with normal feline bone marrow cells, whereas anti-CD33 and anti-CD117 cross-reacted with the blast cells in the bone marrow of two cats with myelodysplastic syndrome, and anti-CD71, anti-235a, anti-41 and anti-42 cross-reacted with immature erythroid cells in a cat with erythroleukaemia. In a feline immunodeficiency virus-positive cat, bone marrow cells were labelled with anti-CD33, anti-14 and anti-45. Anti-CD18, anti-CD14, anti-CD41 and anti-CD61 also reacted with the peripheral blood cells of the healthy cats. The feline haematopoietic progenitors formed colonies in the methylcellulose-based semisolid medium with significant enrichment of colony-forming unit-granulocyte, monocyte and burst-forming unit-erythroid. A panel of six anti-feline mAbs (anti-CD21-like, anti-T lymphocytes, anti-CD172a, anti-granulocyte, anti-CD45-like and anti-CD18) and eight anti-human antibodies (anti-CD71, anti-CD33, anti-CD235a, anti-CD41, anti-CD61, anti-CD117, anti-CD38 and anti-CD34) were used for the immunophenotypic characterisation of the feline bone marrow progenitors. CD45, CD33, CD235a and CD18 were expressed by the feline haematopoietic progenitor cells, with the highest expression level for CD45.
Conflict of interest statement
The authors do not have any potential conflicts of interest to declare.
Figures



Similar articles
-
Comparative multi-color flow cytometric analysis of cell surface antigens in bone marrow hematopoietic progenitors between refractory anemia and aplastic anemia.Leuk Res. 2000 Apr;24(4):359-66. doi: 10.1016/s0145-2126(99)00194-0. Leuk Res. 2000. PMID: 10713334
-
Collection and analysis of hematopoietic progenitor cells from cynomolgus macaques (Macaca fascicularis): assessment of cross-reacting monoclonal antibodies.Am J Primatol. 2003 Sep;61(1):3-12. doi: 10.1002/ajp.10104. Am J Primatol. 2003. PMID: 12966515
-
Immunophenotypic characterization of human bone marrow mast cells. A flow cytometric study of normal and pathological bone marrow samples.Anal Cell Pathol. 1998;16(3):151-9. doi: 10.1155/1998/341340. Anal Cell Pathol. 1998. PMID: 9699944 Free PMC article.
-
Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38- progenitor cells.Blood. 1991 Mar 15;77(6):1218-27. Blood. 1991. PMID: 1705833
-
Identification and comparison of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolor flow cytometry.Blood. 1991 Jun 15;77(12):2591-6. Blood. 1991. PMID: 1710512
Cited by
-
Feline hypertrophic cardiomyopathy: reduced microvascular density and involvement of CD34+ interstitial cells.Vet Pathol. 2022 Mar;59(2):269-283. doi: 10.1177/03009858211062631. Epub 2021 Dec 27. Vet Pathol. 2022. PMID: 34955067 Free PMC article.
-
Anti-CD71 antibody immunohistochemistry in the diagnosis of acute myeloid leukemia, subtype acute erythroid leukemia with erythroid dominance (AML M6-Er), in a retrovirus-negative cat.J Vet Diagn Invest. 2021 Jan;33(1):87-94. doi: 10.1177/1040638720973403. Epub 2020 Nov 22. J Vet Diagn Invest. 2021. PMID: 33225861 Free PMC article.
-
Feline Adenovirus Isolate Shows Silent Nucleotide Alterations, Alternative Receptor/Coreceptor Binding, High Resistance to Disinfectants and Antiviral Drugs, as Well as Immunomodulation.Animals (Basel). 2024 Dec 4;14(23):3502. doi: 10.3390/ani14233502. Animals (Basel). 2024. PMID: 39682467 Free PMC article.
References
-
- Meister RK, Taglinger K, Haverson K, et al. Progress in the discovery and definition of monoclonal antibodies for use in feline research. Vet Immunol Immunopathol 2007; 119: 38–46. - PubMed
-
- Breuer W, Hermanns W, Thiele J. Myelodysplastic syndrome (MDS), acute myeloid leukaemia (AML) and chronic myeloproliferative disorder (CMPD) in cats. J Comp Pathol 1999; 121: 203–216. - PubMed
-
- Hutson C, Rideout B, Pedersen N. Neoplasia associated with feline immunodeficiency virus infection in cats of southern California. J Am Vet Med Assoc 1991; 199: 1357 –1362. - PubMed
-
- Jain NC. Classification of myeloproliferative disorders in cats using criteria proposed by the Animal Leukaemia Study Group: A retrospective study of 181 cases (1969–1992). Comp Haematol Int 1993; 3: 125–134.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

